Abstract
Regulatory factor X4 variant 3 (RFX4_v3) is a recently identified transcription factor specifically expressed in the brain. Gene disruption in mice demonstrated that interruption of a single allele (heterozygous, +/−) prevented formation of the subcommissural organ (SCO), resulting in congenital hydrocephalus, whereas interruption of two alleles (homozygous, −/−) caused fatal failure of dorsal midline brain structure formation. These mutagenesis studies implicated RFX4_v3 in early brain development as well as the genesis of the SCO. Rfx4_v3 deficiency presumably causes abnormalities in brain by altering the expression levels of many genes that are crucial for brain morphogenesis, such as the signaling components in the Wnt, bone morphogenetic protein, and retinoic acid pathways. RFX4_v3 might affect these critical signaling pathways in brain development. Cx3cl1, a chemokine gene highly expressed in brain, was identified as a direct target for RFX4_v3, indicating that RFX4_v3 possesses trans-acting activity to stimulate gene expression. Rfx4_v3 is highly expressed in the suprachiasmatic nucleus and might be involved in regulating the circadian clock. One haplotype in RFX4_v3 gene is linked to a higher risk of bipolar disorder, suggesting that this protein might contribute to the pathogenesis of the disease. This Mini-Review describes our current knowledge about RFX4_v3, an important protein that appears to be involved in many aspects of brain development and disease.
Keywords: regulatory factor X, hydrocephalus, midline, brain development
Regulatory factor X (RFX) members belong to the winged-helix subfamily of helix-turn-helix transcription factors, which share a highly conserved 76-amino-acid DNA binding domain (DBD) that is distinct from any other DBD (Gajiwala et al., 2000). These proteins regulate the expression of their target genes by binding to symmetrical “X-box” consensus sequences (Gajiwala et al., 2000). RFX family members have been identified in a broad range of eukaryotic organisms, including yeast, fungi, nematodes, fruit flies, mice, and humans (Morotomi-Yano et al., 2002).
In mammals, five RFX proteins have been identified, named RFX1–RFX5 (Morotomi-Yano et al., 2002). RFX1, RFX2, and RFX3 are structurally closely related proteins which share the DBD, Q (glutamine-rich), and/or PQ (proline- and glutamine-rich) regions and four additional evolutionarily conserved regions, A, B, C, and dimerization domains (Fig. 1B). These proteins form homodimers or heterodimers through their dimerization domains and then regulate downstream gene expression. RFX3 has been shown to direct nodal cilium development and left-right asymmetry specification (Bonnafe et al., 2004). The functions of RFX1 and RFX2 remain elusive, but they have been implicated in modulating the expression of certain medically important genes, such as the interleukin-5 receptor-α chain (IL-5Rα; Iwama et al., 1999). RFX5 is the most intensively studied family member. It contains the conserved DBD but lacks the PQ/Q, A, B, C, and dimerization domains. Therefore, RFX5 does not appear to interact with RFX5 itself or any other family member but forms a complex with other transcription factors to regulate major histocompatibility complex class II (MHCII) gene expression. Mutations in RFX5 cause the bare lymphocyte syndrome (Reith and Mach, 2001).
Fig. 1.
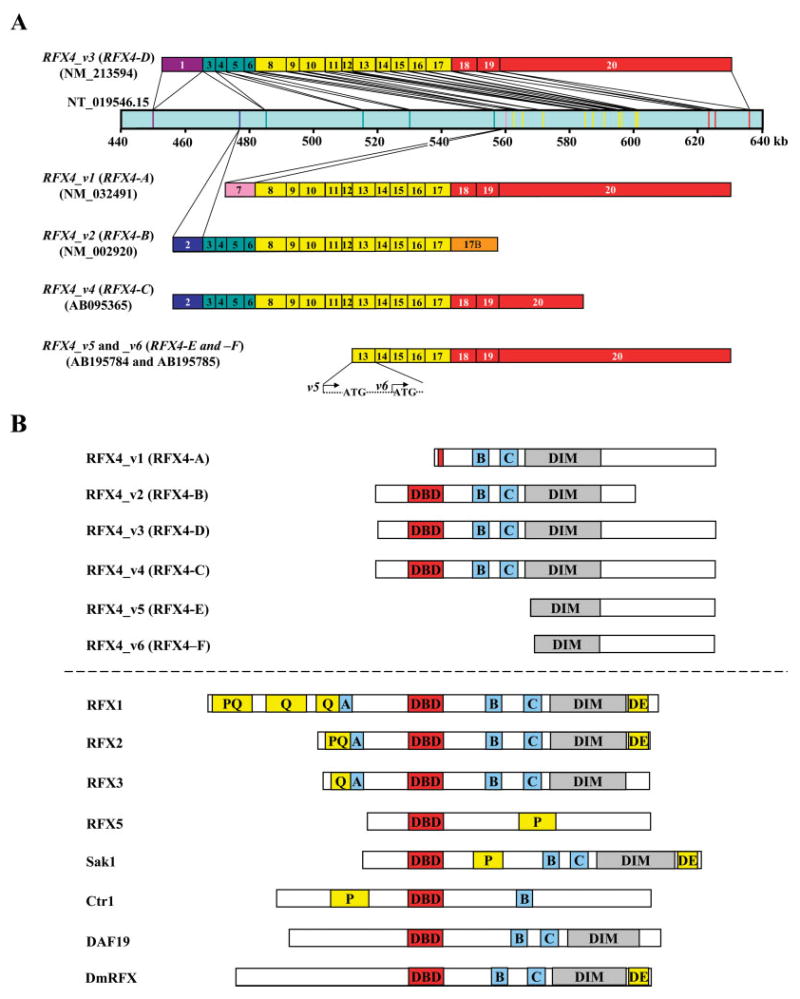
A: The human RFX4 locus and its six known alternative splicing variants (RFX4_v1, _v2, _v3, _v4, _v5, and _v6: also referred to as RFX4-A, -B, -D, -C, -E, and -F, respectively). This figure is a schematic representation of ~200 kb of human genomic sequence from NT_019546.15 and of the positions of the RFX4_v3 exons within this sequence. All the exons are numbered in order from the 5’ to the 3’ ends. Exon 17B (orange) is unique to transcript RFX4_v2. RFX4_v5 starts from the initial base in exon 13, and RFX4_v6 starts from 78 bp downstream of that point. B: Schematic representation of RFX4 proteins and their eukaryotic RFX family members. Six RFX4 isoforms and related RFX members of human (RFX1, RFX2, RFX3, and RFX5), Schizosaccharomyces pombe (Sak1), S. cerevisiae (Crt1), C. elegans (DAF19), and Drosophila melanogaster (DmRFX) are shown. A, B, and C, evolutionarily conserved regions; DE, an acidic region; P and Q, proline- and glutamine-rich regions, respectively; PQ, regions rich in both proline and glutamine; DBD and DIM, RFX-type DNA-binding domains and dimerization domains, respectively.
RFX4 was originally identified in two aberrant cDNA clones derived from a human breast tumor, in which the chimeric molecule contained the amino-terminal half of the estrogen receptor fused to a short RFX-type DBD. This fusion possibly was due to an abnormal chromosomal translocation in breast tumors (Dotzlaw et al., 1992). So far, six isoforms of RFX4 have been identified. In this Mini-Review, we summarize current findings on the structure-function properties of one variant, RFX4_v3, and its potential contributions to brain development and disease.
RFX4_v3 PROTEIN STRUCTURE, RELATED ISOFORMS, AND GENE EXPRESSION IN BRAIN
The first two full-length RFX4 cDNAs were isolated 10 years after the initial identification of the partial cDNA clone (Morotomi-Yano et al., 2002). These two isoforms are expressed specifically in testis and are named RFX4_v1 (for RFX4 transcript variant 1) and RFX4_v2. The third variant of RFX4 was identified serendipitously in our laboratories by insertional mutagenesis. Transgenic mice were generated for cardiac-specific expression of a human epoxygenase gene (Seubert et al., 2004), and one line of these mice developed an unexpected brain phenotype. The transgene was found to be inserted into an intron in the mouse Rfx4 locus and to prevent the expression of a novel brain-specific variant of Rfx4, termed Rfx4_v3 (Blackshear et al., 2003). Three additional RFX4 splice variants have been identified more recently: one isoform appears to be expressed specifically in the testis, and another two transcripts were detected only in gliomas but not in normal tissues (Matsushita et al., 2005; Fig. 1). Interestingly, several RFX4 isoforms lack a DBD (Fig. 1B); whether they can still bind DNA or function as transcription factors is unknown.
The RFX4_v3 transcript variant is the only RFX4 isoform significantly expressed in the fetal and adult brain, and its expression is restricted to brain (Blackshear et al., 2003; Matsushita et al., 2005). Its regional expression pattern is highly dynamic during brain development. At E8.5, Rfx4_v3 mRNA is detected in most of the neural plate but is excluded from the presumptive forebrain region. At E9.5, its expression is mostly restricted to two large regions, the caudal diencephalon/mesencephalon and the spinal cord. By E10.5, the transcript is present throughout the neural tube, and it is also detected in the cerebral cortex. From E12.5 to birth, the neuroepithelium and later the ependyma of most of the neural tube express variable levels of Rfx4_v3 mRNA. It is also strongly expressed in the developing subcommissural organ (SCO) from E14.5 to birth (Blackshear et al., 2003). In the adult mouse brain, Rfx4_v3 mRNA was detectable in all the brain tissues examined: brainstem, midbrain, cerebellum, hippocampus, cerebral cortex, hypothalamus, striatum, frontal lobe, and olfactory bulb (unpublished data). There are also high levels of Rfx4_v3 mRNA in the suprachiasmatic nucleus, the central pacemaker site of the circadian clock (Araki et al., 2004).
RFX4 proteins contain the characteristic DBD, B, C, and dimerization domains but lack the Q/PQ and A regions. Because PQ/Q and A regions play roles in transcriptional activation, it has been suggested that RFX4 has no trans-activation capacity but instead functions as a transcriptional repressor. This hypothesis was supported by chimeric protein experiments: fusions of various portions of the RFX4 protein with the DBD of yeast Gal4 failed to activate a reporter gene under the control of Gal4-binding sites. RFX4 has been shown to interact physically with RFX2, RFX3, and RFX4 itself in transfected cells, so RFX4 was believed to regulate transcription through selective interactions with other RFX members (Morotomi-Yano et al., 2002). However, our experiments indicate that RFX4_v3 can function as a transcriptional activator under certain circumstances. One relevant factor is that the RFX4_v3 protein appears to be located only in the cell nucleus (Araki et al., 2004). In addition, Cx3cl1, a CX3C-type chemokine gene highly expressed in brain (Tarozzo et al., 2003), is down-regulated in the Rfx4_v3 −/− brain (Zhang et al., 2006). Both human and mouse Cx3cl1 proximal promoters contain highly conserved X-boxes, known cis-acting elements for RFX protein binding. RFX4_v3 protein can bind directly to the X-box 1 of the Cx3cl1 promoter in vitro and in vivo and activates expression of this gene through this X-box (Zhang et al., 2006). To date, Cx3cl1 is the only direct transcriptional target identified for RFX4 protein, and the trans-acting activity of RFX4_v3 for this gene suggests that the transcriptional function of RFX4_v3 is more complex than suggested by its protein domain structure.
RFX4_v3 AND NONCOMMUNICATING CONGENITAL HYDROCEPHALUS
Cerebrospinal fluid (CSF) is secreted from the choroid plexus and moves through the ventricular system from the lateral ventricles to the third ventricle via the foramen of Munro, then through the aqueduct of Sylvius to the fourth ventricle. The fourth ventricle opens into the cisterna magna of the subarachnoid space and the central canal of the spinal cord. Hydrocephalus is the net, pathological accumulation of CSF. With the rare exception of CSF overproduction, most types of hydrocephalus are due to an impairment of CSF flow, within either the ventricular system (noncommunicating hydrocephalus) or the subarachnoid space (communicating hydrocephalus; Rodriguez et al., 2001; Picketts, 2006). Noncommunicating hydrocephalus arises from an obstruction of the ventricular system, usually occurring in the narrowed segments. Several factors play a role in the maintenance of CSF flow through the narrow aqueducts. Ventricular ependymal cilia are critical for directing CSF flow, and primary ciliary dyskinesia, a disease characterized by immotile cilia, is associated with hydrocephalus in humans (Afzelius, 2004). The SCO, an ependymal gland located in the dorsocaudal region of the third ventricle at the entrance of the aqueduct of Sylvius, produces Reissner’s fibers, long, threadlike structures elongating and extending through the aqueduct of Sylvius to the central canal of the spinal cord. Both the organ and fibers are important for the patency of the aqueduct. Indeed, malformation of the SCO leads to hydrocephalus (Rodriguez et al., 2001; Picketts, 2006).
In our laboratories, transgenic mice were originally generated to study the cardiac-specific expression of CYP2J2 (Seubert et al., 2004). Surprisingly, one line of mice developed head swelling and rapid neurological decline in young adulthood (Blackshear et al., 2003). Examination of this line of transgenic mice at the time of birth (P0.5) showed that severe hydrocephalus was present in all mice, indicating that the hydrocephalus was congenital. Histological examination of the brains revealed severe hydrocephalus in the anterior brain, with dramatic dilatation of the lateral ventricles. Antibodies specific to Reissner’s fibers strongly and specifically labeled the SCO from the wild-type mice, but this label was generally not detected in the transgenic mice (Fig. 2). Although the SCO appeared to be largely absent in the transgenic mice, other midline structures, such as the pineal body and posterior commissure, were present and appeared to be normal.
Fig. 2.
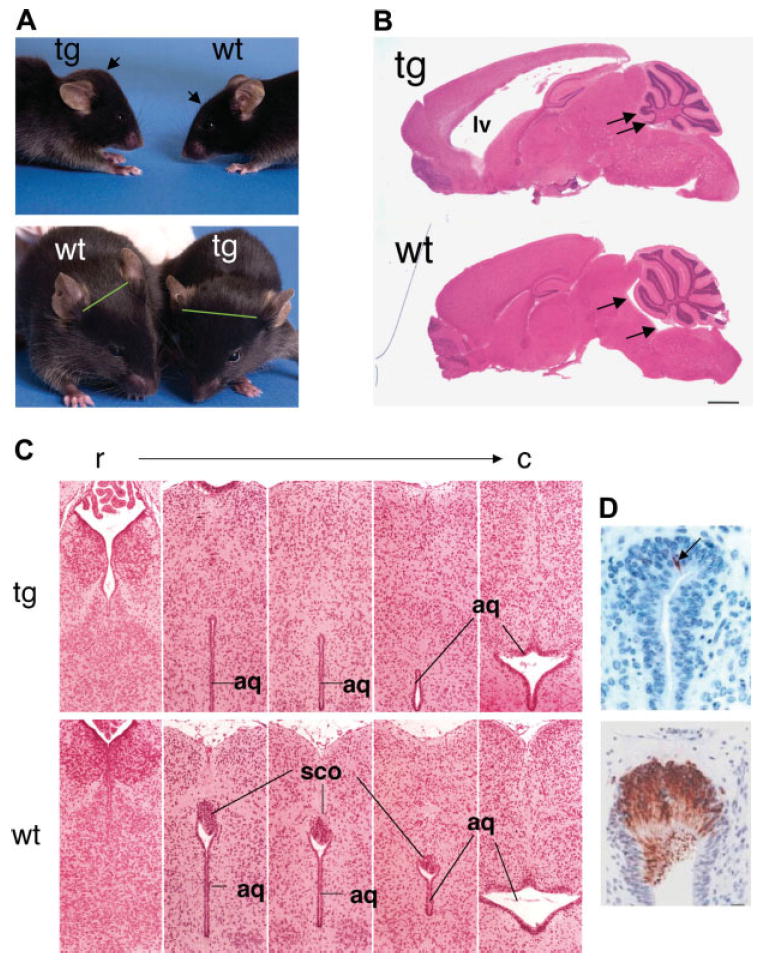
Hydrocephalus in transgenic (Rfx4_v3 +/−) mice. A: Two littermate mice in lateral (top) and frontal (bottom) view at about 2 months of age, showing the increased convexity of the head (arrows) and the lateral displacement of the ears (green lines) in the heterozygous littermate. B: Parasagittal sections, stained with hematoxylin and eosin, of brains from one transgenic and one wild-type littermate, at about 7 weeks of age. The marked dilatation of the lateral ventricles (LV) is obvious in the transgenic mice, without apparent dilatation of the fourth ventricles (arrows). C: Coronal sections in a rostral (r) to caudal (c) direction from P0.5 wild-type and transgenic littermates stained with hematoxylin and eosin, demonstrating the apparent absence of the SCO in the transgenic mouse. aq, Aqueduct of Sylvius. D: Similar sections stained with an antibody to Reissner’s fibers for both wild-type and transgenic brains at P0.5. Adapted from Blackshear et al. (2003) with permission.
The >15-kb CYP2J2 transgene was found to be inserted into the intron between exons 19 and 20 of the Rfx4_v3 gene, within the carboxyl-terminal end of the protein coding region. It presumably interferes with splicing of the final exon and generation of an intact mature mRNA. Interruption of a single allele of Rfx4_v3 (+/−) in the transgenic mice resulted in quantitatively decreased expression of this gene in brain. This in turn was associated with agenesis of the SCO, which was presumably the cause of stenosis of the aqueduct of Sylvius and noncommunicating congenital hydrocephalus. The unusual finding that expression of only a single allele leads to congenital hydrocephalus, at least in mice, suggests that this defect is due either to autosomal dominance or, more likely, to gene dosage effects. The agenesis of the SCO in Rfx4_v3 +/− mice appeared to be the main cause of the hydrocephalus, although the ependymal cilia might also exhibit abnormalities (unpublished data). This is intriguing because RFX transcription factors have been shown to regulate the assembly and function of cilia in nematodes, fruit flies, and mice (Swoboda et al., 2000; Dubruille et al., 2002; Bonnafe et al., 2004), and Rfx3 −/− mice have hydrocephalus associated with abnormal differentiation of ciliated ependymal cells (Baas et al., 2006). Several aspects of the brain phenotype of Rfx4_v3 +/− mice are quite similar to those found in the Rfx3 −/− mice. RFX3 and RFX4 can form heterodimers in vitro, and whether the two proteins can form dimers in vivo is unknown. It will be of interest to determine whether these two genes function in the same or different processes of regulation of SCO and ependymal cilia development. Congenital hydrocephalus affects approximately 1–3 of 1,000 children at birth, and to date only the L1_CAM gene has been linked to this type of hydrocephalus in humans (Weller and Gartner, 2001). We are currently exploring the possibility that some human cases of congenital hydrocephalus are due to abnormalities in the expression or sequence of the RFX4_v3 gene.
RFX4_v3 AND MIDLINE BRAIN STRUCTURE FORMATION
Although all the Rfx4_v3 +/− mice develop hydrocephalus, some mice survive long enough to be interbred to generate −/− mice (Blackshear et al., 2003). Disruption of both Rfx4_v3 alleles severely alters early brain morphogenesis, and all the −/− pups die perinatally. At E12.5, the −/− mice have abnormal doming of the skull and smaller heads compared with their +/+ littermates. In the most rostral part of the head, the +/+ and −/− brains appear similar, both having two lateral ventricles and normal midline brain structure. However, the dorsal structures in the rostral −/− brain are hypoplastic and lack morphological differentiation of medial and paramedial dorsal structures. This abnormality is most striking in the forebrain and midbrain, with loss of midline structures and formation of a single central ventricle. The brains of −/− mice at time of birth are more grossly dysmorphic.
Another group used forward genetic screening to identify mutations that disrupt cortical development in mice (Zarbalis et al., 2004). Ethylnitrosourea (ENU) generally induces a single base pair substitution, and in this study the mice were treated with ENU to generate recessive mutations. In one line of homozygous mice, a morphologically identifiable dorsal midline was absent from the cerebral cortex. The mutation was mapped to the Rfx4 locus, and sequencing revealed a base substitution that changed a leucine residue to a proline within the conserved carboxyl-terminal domain of RFX4’s larger dimerization domain (Katan-Khaykovich et al., 1999), suggesting that RFX4_v3-containing dimers regulate important transcriptional events during cortical midline formation. In the homozygous mouse brain, the defects caused by the missense mutation were very similar to the phenotype generated by insertional mutations in our laboratories, further supporting the importance of RFX4_v3 in the development of midline brain structures.
In our studies, interruption of both Rfx4 alleles resulted in profound failure of dorsal midline structure formation, possibly by interfering with the expression of downstream genes. To search for potential targets for RFX4_v3, we used microarray analysis to identify differentially expressed genes in Rfx4_v3 −/− mouse brains at embryonic day 10.5, before gross structural changes were apparent, and some outlier genes were selected for validation by real-time PCR (Zhang et al., 2006). Many of the confirmed genes encode proteins that are critical for brain morphogenesis, such as the signaling components in the Wnt, bone morphogenetic protein (BMP), and retinoic acid (RA) pathways, and others represent transcription factors important for brain development (Table I). Many of the validated genes contained putative X-box sequences in their proximal promoters (Table I), and further studies will be needed to investigate whether the RFX4_v3 protein regulates these genes through the identified X-boxes. Wnt proteins play diverse roles in both early and later stages of brain development, and mice deficient in Wnt signaling from the cortical hem have defects in growth and patterning of the dorsomedial cerebral cortex (Lee et al., 2000). BMPs are regulators of dorsal forebrain development, and BMP signaling is required locally to pattern the dorsal telencephalic midline (Furuta et al., 1997; Hebert et al., 2002). RA also plays a role in patterning of the forebrain (Halilagic et al., 2003). In the Rfx4_v3 −/− brains, these signaling pathways may be impaired, suggesting that RFX4_v3 might be involved in regulating these signals and may play a general role in controlling dorsal brain patterning. RFX4_v3 also appears to modulate the expression of some critical transcription factors that are important for brain development, such as Rax, Foxa2, Zic1, and Zic3. RFX4_v3 may either directly or indirectly regulate downstream gene expression by controlling the expression levels of these transcription factors. Much future work will be required to understand the nature of the responses of these genes to Rfx4_v3 deficiency and the molecular mechanisms underlying the function of the RFX4_v3 protein in brain morphogenesis.
TABLE I.
Real-time PCR Validation of Selected Genes in the Microarray Outlier List (Fold Change: −/− vs. +/+) and Putative X-box Sequences in 1kb Mouse Promoters of Validated Genes. Adapted From Zhang et al. (2006)
| GenBank ID | Gene | Gene description | Microarraya | Real-time PCRa | X-box sequences |
|---|---|---|---|---|---|
| Wnt signaling | |||||
| NM_009522 | Wnt3a | Wingless-related MMTV integration site 3A | −2.16 ± 0.10 | −16.17 ± 1.74 | (−) cttgctcttggcaac −640 |
| NM_009521 | Wnt3 | Wingless-related MMTV integration site 3 | −1.99 ± 0.88 | −4.93 ± 0.53 | |
| AK052950 | Fzd10 | Frizzled 10 precusor | −1.56 ± 0.12 | −2.41 ± 0.14 | |
| NM_027280 | Nkd1 | RIKEN cDNA 2810434J10 gene | −1.52 ± 0.03 | −1.98 ± 0.32 | (−) tgtcactcgcaact −715 |
| Retinoic acid signaling | |||||
| NM_007811 | Cyp26a1 | cytochrome P450, 26, retinoic acid | 1.41 ± 0.12 | 1.65 ± 0.20 | (−) tggggcttgcaacc −860
(−) cgggactaggaaca −226 |
| BMP signaling | |||||
| NM_010836 | Msx3 | homeo box, msh-like 3 | −1.89 ± 0.28 | −14.73 ± 1.58 | (−) ggccactagcaact −636 |
| Transcription factors (not in the Wnt, RA, or BMP pathways) | |||||
| NM_031166 | Idb4 | inhibitor of DNA binding 4 | 1.38 ± 0.09 | 1.68 ± 0.22 | (−) ggcgccgggcaacc −470 |
| AK014242 | Fez | RIKEN cDNA 3110069A13 gene | 1.50 ± 0.10 | 1.58 ± 0.10 | |
| NM_013833 | Rax | retina and anterior neural fold homeobox | 1.40 ± 0.05 | 1.90 ± 0.19 | (−) atcgctcttagcaac −899 |
| NM_010446 | Foxa2 | forkhead box A2 | −1.59 ± 0.10 | −1.51 ± 0.09 | (+) gtaaccttgaaaca −123 |
| AK034131 | Rfx4_v3 | regulatory factor X, 4 | −1.32 ± 0.06 | −6.81 ± 2.18 | N/A |
| NM_022435 | Sp5 | trans-acting transcription factor 5 | −2.89 ± 0.19 | −3.76 ± 0.43 | (+) tgtcccgagcaacc −148 |
| AK053799 | Bh1 | homeobox protein B-H1 | −2.68 ± 0.21 | −2.98 ± 0.44 | |
| NM_009573 | Zic1 | zinc finger protein of the cerebellum 1 | −1.85 ± 0.07 | −2.20 ± 0.24 | |
| NM_009575 | Zic3 | zinc finger protein of the cerebellum 3 | −1.63 ± 0.04 | −2.08 ± 0.21 | (−) aatagcaggaaaca −850
(−) gaggctgtggggaac −286 |
| Transmembrane proteins (not in the Wnt, RA, or BMP pathways) | |||||
| NM_010052 | Dlk1 | delta-like homolog | 1.40 ± 0.03 | 1.63 ± 0.10 | |
| NM_008516 | Lrrn1 | leucine rich repeat protein 1, neuronal | −1.79 ± 0.11 | −1.70 ± 0.18 | |
| NM_133241 | Mlc1 | Megalencephalic leukoencephalopathy with subcortical cysts 1 homolog. | −1.63 ± 0.11 | −2.28 ± 0.33 | (−) tatagcaggcaact −965
(+) ggacgctgggaact −418 (+) gctgccatgggaac −15 |
| Cytokines | |||||
| NM_009142 | Cx3cl1 | CX3C ligand 1 | −2.05 ± 0.03 | −2.32 ± 0.12 | (+) tgtttcaggaaact −427
(−) ggcgcgatgggaac −171 (+) ggttccctggcaac −122 |
| Other genes | |||||
| NM_138683 | Rspondin | Mus musculus thrombospondin type 1 | −1.64 ± 0.06 | −1.50 ± 0.25 | (−) agacacctgcaacg −593 |
| NM_011707 | Vtn | vitronectin | −1.46 ± 0.04 | −1.69 ± 0.25 | (+) gggaactaggaact −41 |
| AK044492 | EST | adult retina cDNA, clone: A930016D02 | −2.45 ± 0.22 | −2.78 ± 0.78 | (+) gccccaatggaaac −288 |
Mean ± SEM. A negative value indicates decreased expression in −/− vs.+/+, whereas a positive value indicates increased expression. N/A, promoter sequences are not available from the UCSC genome browser. The X-box sequences are identified in either the plus (+) or minus (−) strand, and the positions are shown relative to the transcription start site.
OTHER POTENTIAL FUNCTIONS OF RFX4_v3 IN BRAIN
The suprachiasmatic nucleus (SCN) is located in the hypothalamus immediately above the optic chiasm on either side of the third ventricle. The SCN generates a circadian rhythm of neuronal and hormonal activities, which regulate many different body functions over a 24-hr period, including daily rhythms in locomotor activity, hormone production, and body temperature. Rfx4_v3 message is abundantly expressed in the SCN, suggesting that the function of this gene might be related to the circadian clock. Many circadian genes in the SCN have two characteristics: their mRNAs oscillate during the 24-hr cycle, so the gene expressions are rhythmic; and the gene levels are also induced by acute light exposure during the subjective night, which can shift the circadian clock, but not in the subjective day (Ko and Takahashi, 2006). Rfx4_v3 mRNA was induced by light in a subjective, night-specific manner, although this pattern of gene expression was not reported to be rhythmic (Araki et al., 2004). Further investigation is needed to determine whether this transcription factor is involved in regulating the “master” molecular clock that in turn drives behavioral and molecular rhythms.
Bipolar disorder (BPD), also known as manic-depressive illness, is a serious psychiatric illness that causes shifts in a person’s mood, energy, and ability to function. This disease has a lifetime prevalence of 1%. Independent linkage studies have identified chromosome 12q23-24 as a genomic region that is likely to contain at least one susceptibility locus for BPD (Curtis et al., 2003), and the Rfx4 gene resides on chromosome 12q23.11. BPD is also related to the disruption of circadian rhythms (Wehr et al., 1983), and RFX4_v3 protein might be involved in the SCN function as noted above. A screening of the BPD patients demonstrated an association of BPD with a haplotype at RFX4 derived from markers rs10778502 and ss24735177, suggesting that these two polymorphisms might confer a higher risk of BPD in the general population (Glaser et al., 2005). The associated polymorphisms have no obvious effect on protein structure, whereas investigation of RFX4 brain cDNA tagged by rs10778502 shows evidence for significant allelic differences in gene expression, providing a possible explanation for the pathogenic potential of these mutations. BDP is a complex disease, and it is important to perform an independent confirmatory study to investigate the involvement of RFX4_v3 in BPD.
SUMMARY AND FUTURE DIRECTIONS
RFX4_v3 is a relatively novel protein identified in the CNS, and we are only beginning to understand its role in brain. Insertional mutagenesis studies in mice clearly indicate that this gene is crucial for early brain development and the pathogenesis of hydrocephalus. Whether RFX4_v3 plays similar roles in human is unknown. In the Rfx4_v3 mutant brain, several important signaling pathways are affected. Understanding the regulatory network between RFX4_v3 and these pathways will provide valuable insights into RFX4_v3 function as well as the process of brain morphogenesis in general. Rfx4_v3 −/− mice die perinatally, so the gene’s role in the brain after birth has not been defined. Generating tissue-specific conditional knockout mice might address this issue. RFX4_v3 functions as a transcription factor, and it may associate with other activators/repressors to modulate downstream target genes. Identification of these interacting proteins will help us better to understand RFX4_v3-dependent transcriptional regulation, as will the identification of additional target genes. RFX4_v3 has been implicated in the control of the circadian clock and in susceptibility to BPD; however, more studies are needed to confirm its roles in these physiological and pathological processes.
Acknowledgments
We thank Drs. Jean Harry and Serena Durek for valuable comments on the manuscript.
Contract grant sponsor: Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
Published online 17 May 2007 in Wiley InterScience (www.interscience.wiley.com).
References
- Afzelius BA. Cilia-related diseases. J Pathol. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki R, Takahashi H, Fukumura R, Sun F, Umeda N, Sujino M, Inouye ST, Saito T, Abe M. Restricted expression and photic induction of a novel mouse regulatory factor X4 transcript in the suprachiasmatic nucleus. J Biol Chem. 2004;279:10237–10242. doi: 10.1074/jbc.M312761200. [DOI] [PubMed] [Google Scholar]
- Baas D, Meiniel A, Benadiba C, Bonnafe E, Meiniel O, Reith W, Durand B. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci. 2006;24:1020–1030. doi: 10.1111/j.1460-9568.2006.05002.x. [DOI] [PubMed] [Google Scholar]
- Blackshear PJ, Graves JP, Stumpo DJ, Cobos I, Rubenstein JL, Zeldin DC. Graded phenotypic response to partial and complete deficiency of a brain-specific transcript variant of the winged helix transcription factor RFX4. Development. 2003;130:4539–4552. doi: 10.1242/dev.00661. [DOI] [PubMed] [Google Scholar]
- Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol. 2004;24:4417–4427. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D, Kalsi G, Brynjolfsson J, McInnis M, O’Neill J, Smyth C, Moloney E, Murphy P, McQuillin A, Petursson H, Gurling H. Genome scan of pedigrees multiply affected with bipolar disorder provides further support for the presence of a susceptibility locus on chromosome 12q23-q24, and suggests the presence of additional loci on 1p and 1q. Psychiatr Genet. 2003;13:77–84. doi: 10.1097/01.ypg.0000056684.89558.d2. [DOI] [PubMed] [Google Scholar]
- Dotzlaw H, Alkhalaf M, Murphy LC. Characterization of estrogen receptor variant mRNAs from human breast cancers. Mol Endocrinol. 1992;6:773–785. doi: 10.1210/mend.6.5.1603086. [DOI] [PubMed] [Google Scholar]
- Dubruille R, Laurencon A, Vandaele C, Shishido E, Coulon-Bublex M, Swoboda P, Couble P, Kernan M, Durand B. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development. 2002;129:5487–5498. doi: 10.1242/dev.00148. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Gajiwala KS, Chen H, Cornille F, Roques BP, Reith W, Mach B, Burley SK. Structure of the winged-helix protein hRFX1 reveals a new mode of DNA binding. Nature. 2000;403:916–921. doi: 10.1038/35002634. [DOI] [PubMed] [Google Scholar]
- Glaser B, Kirov G, Bray NJ, Green E, O’Donovan MC, Craddock N, Owen MJ. Identification of a potential bipolar risk haplotype in the gene encoding the winged-helix transcription factor RFX4. Mol Psychiatry. 2005;10:920–927. doi: 10.1038/sj.mp.4001689. [DOI] [PubMed] [Google Scholar]
- Halilagic A, Zile MH, Studer M. A novel role for retinoids in patterning the avian forebrain during presomite stages. Development. 2003;130:2039–2050. doi: 10.1242/dev.00423. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Iwama A, Pan J, Zhang P, Reith W, Mach B, Tenen DG, Sun Z. Dimeric RFX proteins contribute to the activity and lineage specificity of the interleukin-5 receptor alpha promoter through activation and repression domains. Mol Cell Biol. 1999;19:3940–3950. doi: 10.1128/mcb.19.6.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan-Khaykovich Y, Spiegel I, Shaul Y. The dimerization/repression domain of RFX1 is related to a conserved region of its yeast homologues Crt1 and Sak1: a new function for an ancient motif. J Mol Biol. 1999;294:121–137. doi: 10.1006/jmbi.1999.3245. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Uenaka A, Ono T, Hasegawa K, Sato S, Koizumi F, Nakagawa K, Toda M, Shingo T, Ichikawa T, Noguchi Y, Tamiya T, Furuta T, Kawase T, Date I, Nakayama E. Identification of glioma-specific RFX4-E and -F isoforms and humoral immune response in patients. Cancer Sci. 2005;96:801–809. doi: 10.1111/j.1349-7006.2005.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi-Yano K, Yano K, Saito H, Sun Z, Iwama A, Miki Y. Human regulatory factor X 4 (RFX4) is a testis-specific dimeric DNA-binding protein that cooperates with other human RFX members. J Biol Chem. 2002;277:836–842. doi: 10.1074/jbc.M108638200. [DOI] [PubMed] [Google Scholar]
- Picketts DJ. Neuropeptide signaling and hydrocephalus: SCO with the flow. J Clin Invest. 2006;116:1828–1832. doi: 10.1172/JCI29148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Oksche A, Montecinos H. Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc Res Techniq. 2001;52:573–590. doi: 10.1002/1097-0029(20010301)52:5<573::AID-JEMT1042>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol Cell. 2000;5:411–421. doi: 10.1016/s1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- Tarozzo G, Bortolazzi S, Crochemore C, Chen SC, Lira AS, Abrams JS, Beltramo M. Fractalkine protein localization and gene expression in mouse brain. J Neurosci Res. 2003;73:81–88. doi: 10.1002/jnr.10645. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Sack D, Rosenthal N, Duncan W, Gillin JC. Circadian rhythm disturbances in manic-depressive illness. Fed Proc. 1983;42:2809–2814. [PubMed] [Google Scholar]
- Weller S, Gartner J. Genetic and clinical aspects of X-linked hydrocephalus (L1 disease): mutations in the L1CAM gene. Hum Mutat. 2001;18:1–12. doi: 10.1002/humu.1144. [DOI] [PubMed] [Google Scholar]
- Zarbalis K, May SR, Shen Y, Ekker M, Rubenstein JL, Peterson AS. A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development. PLoS Biol. 2004;2:E219. doi: 10.1371/journal.pbio.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Stumpo DJ, Graves JP, DeGraff LM, Grissom SF, Collins JB, Li L, Zeldin DC, Blackshear PJ. Identification of potential target genes for RFX4_v3, a transcription factor critical for brain development. J Neurochem. 2006;98:860–875. doi: 10.1111/j.1471-4159.2006.03930.x. [DOI] [PubMed] [Google Scholar]


