Abstract
A simple strategy for linking biomolecules to porous Si surfaces and detecting peptide/drug binding is described. Porous Si is prepared using an electrochemical etch and then thermally oxidized by heating in ambient atmosphere. Bovine serum albumin (BSA) is then non-covalently adsorbed to the inner pore walls of the porous Si oxide (PSiO2) matrix. The BSA layer is used as a linker for covalent attachment of the peptide Ac-L-Lysine-D-Alanine-D-Alanine (KAA) using published bioconjugation chemistry. BSA-coated surfaces functionalized with KAA display specificity for the glycopeptide vancomycin while resisting adsorption of non-specific reagents. While the biomolecule attachment strategy reported here is used to bind peptides, the scheme can be generalized to the linking of any primary amine-containing molecule to PSiO2 surfaces.
1. Introduction
Measurement of biomolecular interactions is of great interest for pharmaceutical applications, environmental sensing, and the fundamental understanding of biological processes. A number of label-free techniques have been developed to address the need to directly monitor biomolecular interactions, including surface plasmon resonance (SPR) [1–4] and reflectance spectroscopy [5, 6]. Porous Si (PSi) is an attractive substrate for biosensing because it can be prepared with pore sizes that span biologically relevant length scales [7–9], it has a high surface area, and various versatile chemistries for attachment of bio-molecules have been developed [10–19]. Protein and DNA binding has previously been demonstrated using interferometry, a technique that utilizes changes in the white-light reflectance spectrum to monitor binding of biomolecules within or at the surface of the porous nanostructure [18–21].
In this work, we demonstrate a new attachment strategy to immobilize biomolecules on oxidized porous Si. Bovine serum albumin (BSA) is first adsorbed to the inner pore walls of an oxidized porous Si (PSiO2) surface and then utilized as a linker for attachment of a target probe for sensing the biomolecule of interest. We chose the binding of vancomycin, an important glycopeptide-based antibiotic, to the surface-immoblized tripeptide Ac-L-Lysine-D-Alanine-D-Alanine since inexpensive methods for the determination of biologically relevant molecular binding to drug candidates are of particular importance to the pharmaceutical industry.
2. Experimental
Porous Si (PSi) was prepared from single crystal, highly doped (ρ = 9 × 10−4 Ω cm) p-type silicon wafers, which were etched for 20 seconds at a current density of 750 mA/cm2 in a 3:1 (by vol.) 49% aqueous HF:ethanol electrolyte (CAUTION: HF is extremely harmful; use care when working with this chemical!). The high current density was required to generate pores large enough to admit appreciable amounts of protein. Samples were rinsed in ethanol to remove the remaining HF, followed by a rinse in hexane to lower the surface tension and prevent cracking of the PSi film upon drying. The PSi samples were then heated for one hour at 800 °C in a tube furnace (Lindberg Blue) under ambient atmosphere, resulting in completely oxidized porous silicon (PSiO2) films.
An overview of the preparation scheme and principle of sensor modality is presented in Fig. 1. Bovine serum albumin (BSA) was adsorbed onto the pore surface by immersing the PSiO2 chip in a 4 mg/mL BSA (Aldrich, > 95% purity) in pH 4.0 buffer (Fisher Scientific) solution for 4 hours. The samples were rinsed with pH 4.0 phthalate buffer and then soaked in phosphate buffered saline (PBS, pH 7.4) (Calbiochem) overnight to remove weakly-bound BSA from the PSiO2 surface. Ac-L-Lysine-D-Alanine-D-Alanine (KAA) and Ac-L-Lysine-D-Lactone-D-Alanine (K-Lac-A) were custom-synthesized and tested for purity by nuclear magnetic resonance and mass spectrometry. KAA and K-Lac-A were covalently attached to carboxylic acid functional groups of the BSA molecule through standard bioconjugate chemistry [22]. Specifically, the carboxylic acid groups were first activated by immersing the BSA-coated PSiO2 chip in a solution of 15 mM N-hydroxysulfosuccinimide sodium salt (Sulfo-NHS) (Fluka) and 17 mM N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDAC) (Aldrich) in pH 6.0 buffer (Fisher) for 15 min. The sulfo-NHS terminated, BSA-coated chip was then immersed in a solution of 2 mg/mL KAA or K-Lac-A in PBS for 2–4 h, resulting in covalent attachment through the formation of amide bonds between the activated carboxylic acid group of BSA and the primary amine of KAA or K-Lac-A. Remaining sulfo-NHS was removed by dosing the surface with a 0.1 M solution of ethanola-mine for 15 minutes.
Fig. 1.
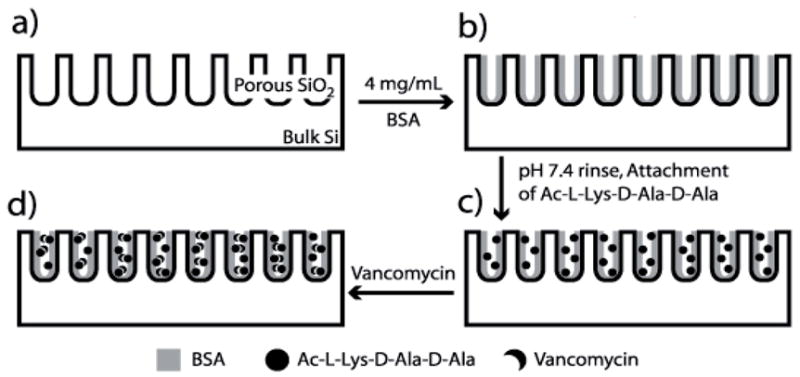
Method for linking biomolecules to BSA-coated porous SiO2. (a) A PSiO2 film is prepared. (b) The sample is submerged in a solution of BSA in pH 4.0 buffer, and the pore walls become coated. (c) Excess BSA is rinsed off in PBS buffer (pH 7.4) and the peptide KAA is attached to the adsorbed protein. (d) Vancomycin is introduced and binds to the attached KAA.
Protein and vancomycin adsorption was measured by monitoring changes in the optical thickness (OT) of the sample film with a reflectance spectrometer as previously described [21]. Briefly, a bifurcated fiber optic cable was used to couple a tungsten white-light source and a charge-coupled device spectrometer (Ocean Optics S-2000) via microscope optics focused on a spot of ~1 mm diameter on the PSiO2 chip. Spectra were collected every thirty seconds. A Hanning window and a fast fourier transform (FFT) was applied to the raw frequency (nm−1) vs. intensity reflectance spectrum, resulting in a single peak with a maximum that corresponded to the optical thickness of the porous film [18, 21]. Samples were maintained in a custom flow cell [21] through which the various dosing solutions were passed.
3. Results and discussion
The goal of this work was to develop a simple general method for preparing porous Si interferometer surfaces to be utilized for sensing biomolecule/drug binding. We chose to study vancomycin/KAA binding due to the importance of understanding how drug candidates (e.g.; antibiotics) interact with biologically relevant molecules (e.g.; specific peptide sequences). For this work, we chose to use porous Si surfaces that have been completely oxidized to eliminate the likelihood of degradation by oxidants such as water and oxygen. A simplified method for attaching a capture probe to an oxidized porous Si (PSiO2) film through non-covalent adsorption has recently been developed for measuring protein binding [23, 24]. The work reported here is a modification of the non-covalent adsorption strategy [23, 24] in which the available functional groups of the adsorbed protein are used to attach the capture probe.
The choice of protein for this study was based on the requirement of strong non-covalent adsorption at physiological pH and the availability of sufficient concentration of carboxyl groups for further bio-molecule attachment. There are many carboxylic acid groups present in a BSA molecule [25], and therefore a significant number of binding sites should remain accessible for attachment of the KAA peptide if BSA adsorbs into the PSiO2 film. Figure 2 demonstrates that dosing BSA in pH 4.0 buffer leads to a significant shift in OT that can be attributed to protein entering the pores [18]. Rinsing the BSA-coated surface with pH 4 buffer does not lead to a significant reduction in OT, indicating that BSA remains adsorbed to the PSiO2 pore walls. Since the purpose of this study is to test drug/biomolecule interactions at physiological pH, PBS buffer (pH = 7.4) was used as the analyte matrix. When pure PBS is introduced, a decrease in OT compared to the pH 4 condition is observed, indicating that some of the more weakly-bound BSA is removed. However, after approximately forty minutes of continuous flow, OT stabilizes at a value ~170 nm higher than the original (pre-BSA) baseline, indicating that a significant amount of BSA remains strongly adsorbed in the PSiO2 film.
Fig. 2.
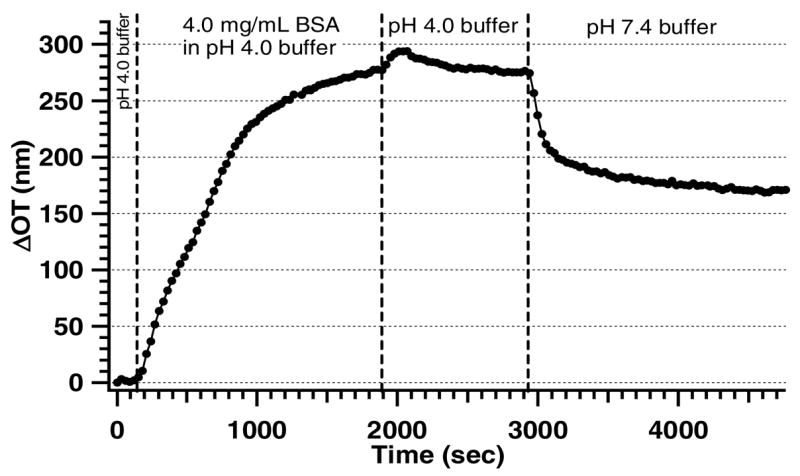
Loading BSA into a PSiO2 film, monitored by optical reflectance interferometry. After establishing a baseline with pH 4.0 buffer, a solution of BSA is introduced into the flow cell, resulting in a marked increase in optical thickness due to adsorption to the inner pore walls of the PSiO2 surface. The PSiO2 sample is then rinsed with pH 4.0 buffer followed by PBS buffer (pH 7.4) to remove any weakly physisorbed BSA from the surface.
Figure 3 illustrates the changes in OT observed for (a) a BSA-coated PSiO2 chip, (b) a BSA-coated chip functionalized with K-Lac-A (BSA/K-Lac-A), and (c) a BSA-coated PSiO2 chip functionalized with KAA (BSA/KAA), each dosed with a 16 μM solution of vancomycin. No change in optical thickness is observed when the BSA-coated (Fig. 3a) and the BSA/K-Lac-A (Fig. 3b) chips are dosed with 16 μM vancomycin. K-Lac-A was used as a control, because it is known to inhibit binding of vancomycin [28]. Additional experiments in which BSA-coated and BSA/KAA-terminated PSiO2 surfaces are exposed to50 μM erythromycin yield plots similar to those shown in Fig. 3a and b in which no change in OT is observed. Finally, an ethanolamine-modified/BSA-coated PSiO2 (BSA/ethanolamine) sample dosed with vancomycin does not lead to a significant change in OT. However, when a BSA/KAA chip (Fig. 3c) is dosed with 16 μM vancomycin, a shift in OT of ~5 nm is observed, while subsequent rinsing with PBS buffer leads to a slow decrease in OT.
Fig. 3.
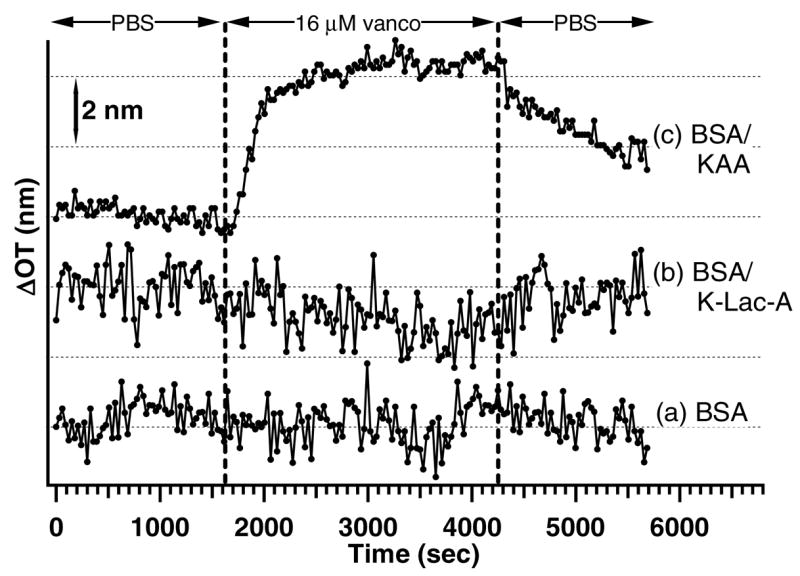
Vancomycin sensing experiments. 16 μM vancomycin was dosed on (a) a BSA-coated PSiO2 surface with no target probe attached, (b) a BSA-coated, K-Lac-A-modified PSiO2 surface (BSA/K-Lac-A), and (c) a BSA-coated, KAA-terminated sample (BSA/KAA). Only the BSA/KAA sample (c) results in a change in OT.
There are several pieces of data that demonstrate the PSiO2-based sensor design described here can be used to specifically measure drug/peptide binding. Dosing with vancomycin leads to a shift in OT (~5 nm) for the BSA/KAA surface but not the BSA-coated (Fig. 3a), the BSA/K-Lac-A (Fig. 3b), or the BSA/ethanolamine (not shown) surfaces, suggesting that the shift is due to specific binding. The fact that no shift is observed when the BSA/K-Lac-A or BSA/ethanolamine chips are dosed with vancomycin indicates that covalent modification of the adsorbed BSA molecule does not lead to inherent changes in surface chemistry that cause non-specific binding. Also, dosing the BSA/KAA surface with erythromycin does not lead to an appreciable shift in OT (not shown), further supporting the idea that the change in OT for vancomycin dosed on a BSA/KAA surface is due to a specific binding event. Moreover, the slow decrease in OT for the vancomycin-dosed KAA surface caused by rinsing with PBS buffer is consistent with removal of a weakly bound molecule from the PSiO2 surface. A shift in OT for vancomycin dosed on a BSA/KAA but not a BSA/K-Lac-A chip is consistent with the fact that KAA is a tripeptide known to bind to the peptide ring of vancomycin [26, 27] while K-Lac-A inhibits vancomycin binding [28]. Therefore, based on the presented data, we conclude that the shift in OT observed when a BSA/KAA surface is dosed with vancomycin can be attributed to specific binding, demonstrating that the strategy used here for attaching biomolecules to PSiO2 films through a non-covalently bound linker protein represents a simple and effective method for interferometric bionsensing.
4. Conclusion
We report the use of a non-covalently adsorbed protein (BSA) on an oxidized porous Si (PSiO2) surface as a linker for the attachment of the peptide L-Lysine-D-Alanine-D-Alanine (KAA). Interferometry shows that a KAA-treated PSiO2 surface specifically binds vancomycin and the BSA linker prevents non-specific binding. The non-covalent/covalent biomolecule attachment strategy reported here should be generally applicable for attaching any molecule containing primary amines to oxidized Si surfaces, useful for studying biomolecular interactions.
Acknowledgments
The authors acknowledge financial support from the National Cancer Institute of the National Institutes of Health (Contract No. N01-C0-37117), and the Air Force Office of Scientific Research (Grant# F49620-02-1-0288). MJS is a member of the Moores UCSD Cancer Center and the UCSD NanoTUMOR Center under which this research was conducted and partially supported by NIH grant U54 CA 119335. LAP acknowledges financial support from the Jacobs School of Engineering Graduate Fellowship. MPS thanks the La Jolla Interfaces in Science program, funded by the Burroughs Wellcome Fund for a post-doctoral fellowship.
References
- 1.Liedberg B, Nylander C, Lundstrom I. Sens Actuators. 1983;4:299. [Google Scholar]
- 2.Liedberg B, Nylander C, Lundstrom I. Biosens Bioelectron. 1995;10:R1. doi: 10.1016/0956-5663(95)96965-2. [DOI] [PubMed] [Google Scholar]
- 3.Homola J, Yee SS, Gauglitz G. Sens Actuators B Chem. 1999;54:3. [Google Scholar]
- 4.Homola J. Anal Bioanal Chem. 2003;377:528. doi: 10.1007/s00216-003-2101-0. [DOI] [PubMed] [Google Scholar]
- 5.Brecht A, Gauglitz G, Polster J. Biosens Bioelectron. 1993;8:387. [Google Scholar]
- 6.Brecht A, Gauglitz G. Sens Actuators B. 1997;38:1. [Google Scholar]
- 7.Tinsley-Bown AM, Canham LT, Hollings M, Anderson MH, Reeves CL, Cox TI, Nicklin S, Squirrell DJ, Perkins E, Hutchinson A, Sailor MJ, Wun A. phys stat sol (a) 2000;182:547. [Google Scholar]
- 8.Collins BE, Dancil KP, Abbi G, Sailor MJ. Adv Funct Mater. 2002;12:187. [Google Scholar]
- 9.Anglin EJ, Schwartz MP, Ng VP, Perelman LA, Sailor MJ. Langmuir. 2004;20:11264. doi: 10.1021/la048105t. [DOI] [PubMed] [Google Scholar]
- 10.Sailor MJ, Lee EJ. Adv Mater. 1997;9:783. [Google Scholar]
- 11.Buriak JM. Chem Rev. 2002;102:1272. doi: 10.1021/cr000064s. [DOI] [PubMed] [Google Scholar]
- 12.Hart BR, Letant SE, Kane SR, Hadi MZ, Shields SJ, Reynolds JG. Chem Commun. 2003;3:322. doi: 10.1039/b209453c. [DOI] [PubMed] [Google Scholar]
- 13.Tsargorodskaya A, Nabok AV, Ray AK. Nanotechnology. 2004;15:703. [Google Scholar]
- 14.Schwartz MP, Cunin F, Cheung RW, Sailor MJ. phys stat sol (a) 2005;202:1380. [Google Scholar]
- 15.De Stefano L, Moretti L, Lamberti A, Longo O, Rocchia M, Rossi AM, Arcari P, Rendina I. IEEE Trans Nanotechnol. 2004;3:49. [Google Scholar]
- 16.Chan S, Fauchet PM, Li Y, Rothberg LJ, Miller BL. phys stat sol (a) 2000;182:541. [Google Scholar]
- 17.Xia B, Xiao SJ, Guo DJ, Wang J, Chao J, Liu HB, Pei J, Chen YQ, Tang YC, Liu JN. J Mater Chem. 2006;16:570. [Google Scholar]
- 18.Dancil KPS, Greiner DP, Sailor MJ. J Am Chem Soc. 1999;121:7925. [Google Scholar]
- 19.Lin VSY, Motesharei K, Dancil KS, Sailor MJ, Ghadiri MR. Science. 1997;278:840. doi: 10.1126/science.278.5339.840. [DOI] [PubMed] [Google Scholar]
- 20.Pacholski C, Sartor M, Sailor MJ, Cunin F, Miskelly GM. J Am Chem Soc. 2005;127:11636. doi: 10.1021/ja0511671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janshoff A, Dancil KPS, Steinem C, Greiner DP, Lin VSY, Gurtner C, Motesharei K, Sailor MJ, Ghadiri MR. J Am Chem Soc. 1998;120:12108. [Google Scholar]
- 22.Hermanson GT. Bioconjugate Techniques. Academic Press, Inc; San Diego: 1996. [Google Scholar]
- 23.Pacholski C, Yu C, Miskelly GM, Godin D, Sailor MJ. J Am Chem Soc. 2006;128:4250. doi: 10.1021/ja056702b. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz MP, Yu C, Migliori B, Alvarez SD, Godin D, Chao L, Sailor MJ. phys stat sol (a) 2007;204:1444. this issue. [Google Scholar]
- 25.Foster JF. In: Albumin Structure, Function and Uses. Rosenoer VM, Oratz M, Roths-child MA, editors. Pergamon; Oxford: 1977. p. 53. [Google Scholar]
- 26.Rao JH, Lahiri J, Weis RM, Whitesides GM. J Am Chem Soc. 2000;122:2698. [Google Scholar]
- 27.Rao JH, Lahiri J, Isaacs L, Weis RM, Whitesides GM. Science. 1998;280:708. doi: 10.1126/science.280.5364.708. [DOI] [PubMed] [Google Scholar]
- 28.Arciola CR, Campoccia D, Montanaro L. Biomaterials. 2002;23:1495. doi: 10.1016/s0142-9612(01)00275-7. [DOI] [PubMed] [Google Scholar]


