Figure 3.
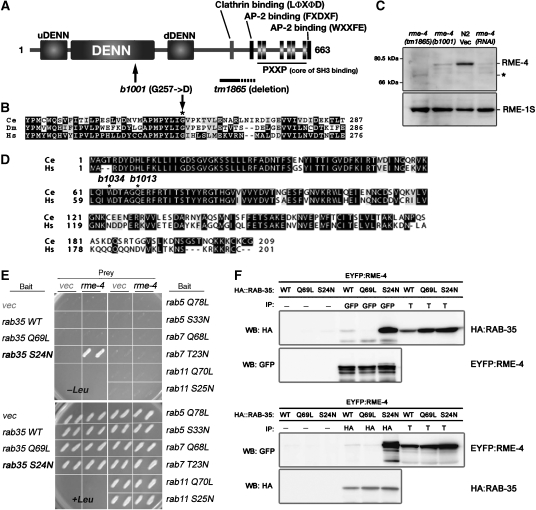
RME-4 is a novel protein containing a DENN domain and physically interacts with RME-5/RAB-35. (A) Domain structure of RME-4, showing the N-terminal DENN domain accompanied by the uDENN and dDENN domains. In addition, RME-4 contains AP-2-binding motifs, a clathrin-binding motif, and a cluster of SH3-binding motifs all located in the C-terminal half of the protein. Mutations identified in rme-4 alleles are also shown. (B) Amino-acid alignment of the highly conserved region of the DENN domain from the human (Hs), C. elegans (Ce), and fly (Dm) RME-4 homologues. In rme-4(b1001), glycine 257 (marked with an asterisk) is mutated. (C) Detection of RME-4 protein. Total lysates were prepared from rme-4 mutant worms and N2 wild-type worms treated with RNAi of either control vector or rme-4. They were examined by immunoblotting using anti-RME-4 antibody. The same membrane was also probed with anti-RME-1 antibody. The asterisk indicates the mutant RME-4(tm1865) protein containing a partial deletion. (D) RME-5 is the worm Rab35 orthologue. Amino-acid alignment of worm RME-5/RAB-35 (Ce) and human Rab35 (Hs). Two alleles of rme-5 contain nonsense mutations at the indicated positions. (E) Yeast two-hybrid interaction between RME-4 and RAB-35. Full-length RME-4 was expressed in a yeast reporter strain as a fusion with the transcriptional activation domain of B42 (prey). C. elegans RAB-35, RAB-5, RAB-7, RAB-11, and their mutant forms were expressed in the same yeast cells as fusions with the DNA binding domain of LexA (bait). Interaction between bait and prey was tested using LEU2 (shown) and β-galactosidase (not shown) reporter assays. (F) Co-immunoprecipitation of EYFP–RME-4 and mRFPCherryHA–RAB-35 or their mutant forms. Total lysates were prepared from worms co-expressing EYFP–RME-4 and mRFPCherryHA–RAB-35 or its mutant forms and subjected to immunoprecipitation with anti-GFP antibody (upper panel) or anti-HA antibody (lower panel). Precipitants were probed on immunoblots with anti-HA and anti-GFP antibodies. T (total lysate) equals approximately 4% of the total input into the assay.