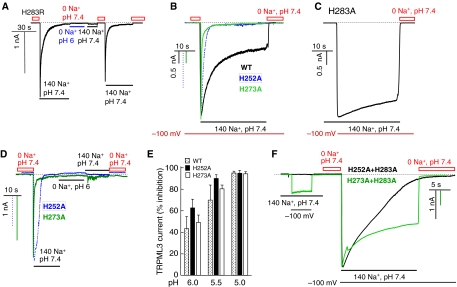
Figure 4.
Effect of H252, H273 and H283R on regulation of TRPML3 by H+e-cyto. (A) The H283R mutation markedly increases the rate of TRPML3 inactivation and TRPML3(H283R) is inhibited by H+e-cyto. The dark dashed line in this and the other panels indicates the 0 current level. The grey line marks the period of incubation with 0 Na+, pH 6. (B) Na+ current was measured in cells expressing wild-type TRPML3 (black trace), TRPML3(H252A) (dashed trace) or TRPML3(H273A) (grey trace). Current measurement was initiated by clamping the membrane potential to −100 mV. Preincubation of wild-type, H252A and H273A TRPML3 in Na+-free solution resulted in full activation of the current. However, the TRPML3 current inactivated along a bi-exponential time course, with fast inactivation that was completed in 10–20 s and a slow inactivation that took a longer time (minutes). The current of the H252A and H273A mutants showed only fast inactivation. (C) The spontaneously active TRPML3(H283A) current was measured in cells continuously bathed in solution containing 140 mM Na+. Current is observed when switching the holding membrane potential from 0 to −100 mV. (D) TRPML3(H252A) (dashed trace) and TRPML3(H273A) (grey trace) activity is inhibited by H+e-cyto. (E) Dependence of the inhibition of the TRPML3(H252A) and TRPML3(H273A) current on H+e-cyto was measured at a holding potential of −100 mV. The cells were exposed to Na+-free medium, pH 7.4, to determine the control current and then to Na+-free medium of the indicated pHo. After exposure to different Na+-free media of the indicated pHo, the current was measured in media containing 140 mM Na+, pH 7.4. (F) Properties of the double mutants TRPML3(H252,283A) (grey trace) and TRPML3(H273,283A) (dark trace). The spontaneous activity was measured by clamping the membrane potential to −100 mV. The cells were then incubated in Na+-free solution, pH 7.4, and the current was measured by clamping the membrane potential to −100 mV in Na+-containing solution, pH 7.4. Note that the H283A mutation markedly reduces and slows down channel inactivation of H252A and H273A. A full-colour version of this figure is available at The EMBO Journal Online.