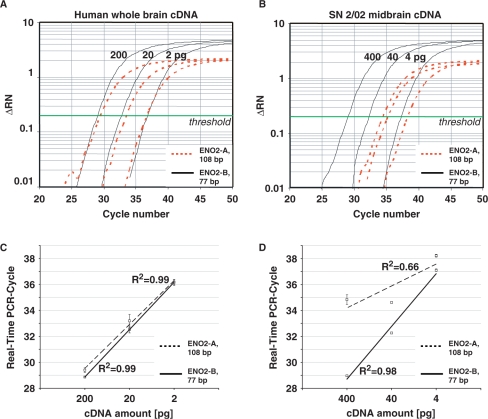
Figure 4.
Effects of RNA integrity and PCR-assay size on real-time quantitative PCR performance. Serial dilutions of high-quality whole brain cDNA (200, 20 and 2 pg; Clontech), and cDNA derived from partly degraded midbrain RNA (400, 40 and 4 pg; brain SN 2/02, RIN = 6.1) were used as templates for ENO2 real-time PCR, employing two different assays for ENO2 with large (ENO2-A, 108 bp) and small (ENO2-B, 77 bp) amplicon size (A and C) With high-quality cDNA as templates, amplification curves (A) and slopes (amplification-efficiencies) of standard curves were similar for both assays (Δ RN: relative fluorescence, normalized to internal fluorescence marker ROX) (C). (B and D) With cDNA from partly degraded RNA as templates, amplification curves (B) and slopes of standard curves (D) were similar as for high-quality cDNA only when using the small amplicon size assay ENO2-B. The larger ENO2-A assay did not allow the generation of a respective reliable standard curve and was not suited for RT–PCR quantification of SN 2/02 RNA sample.