Abstract
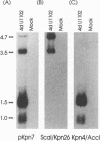
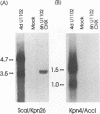
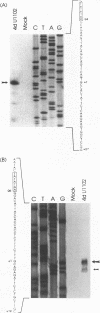
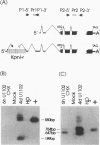
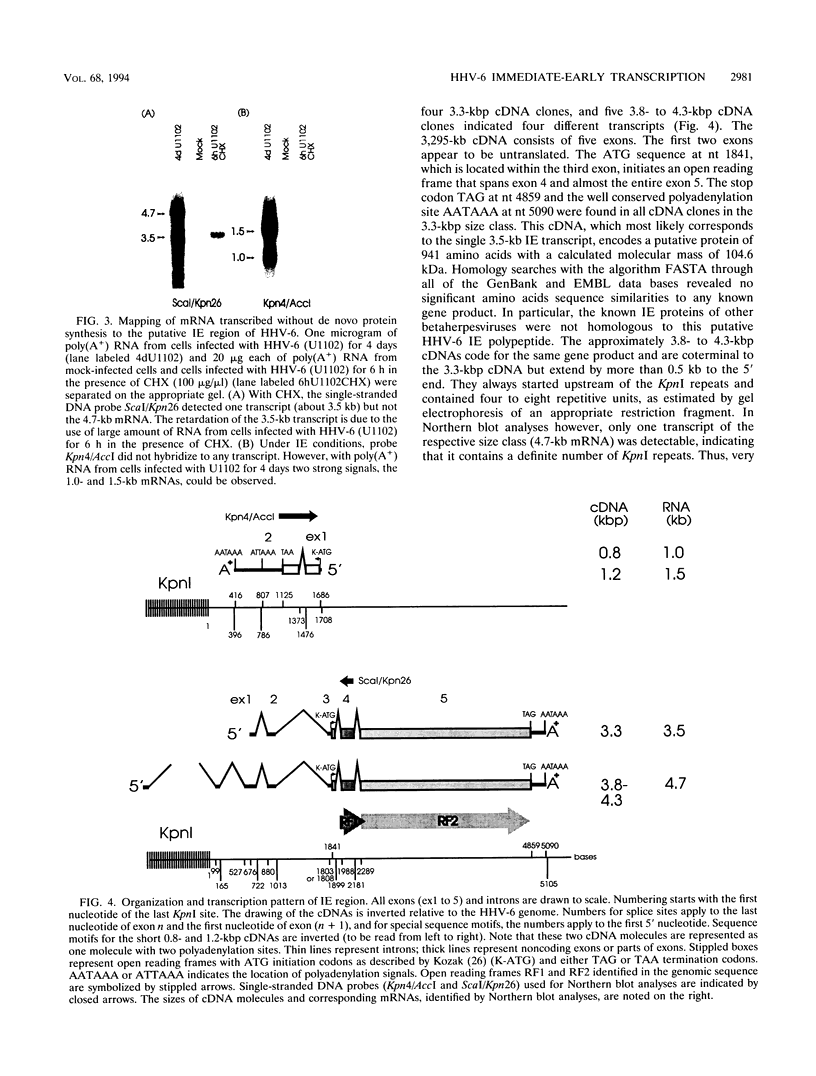
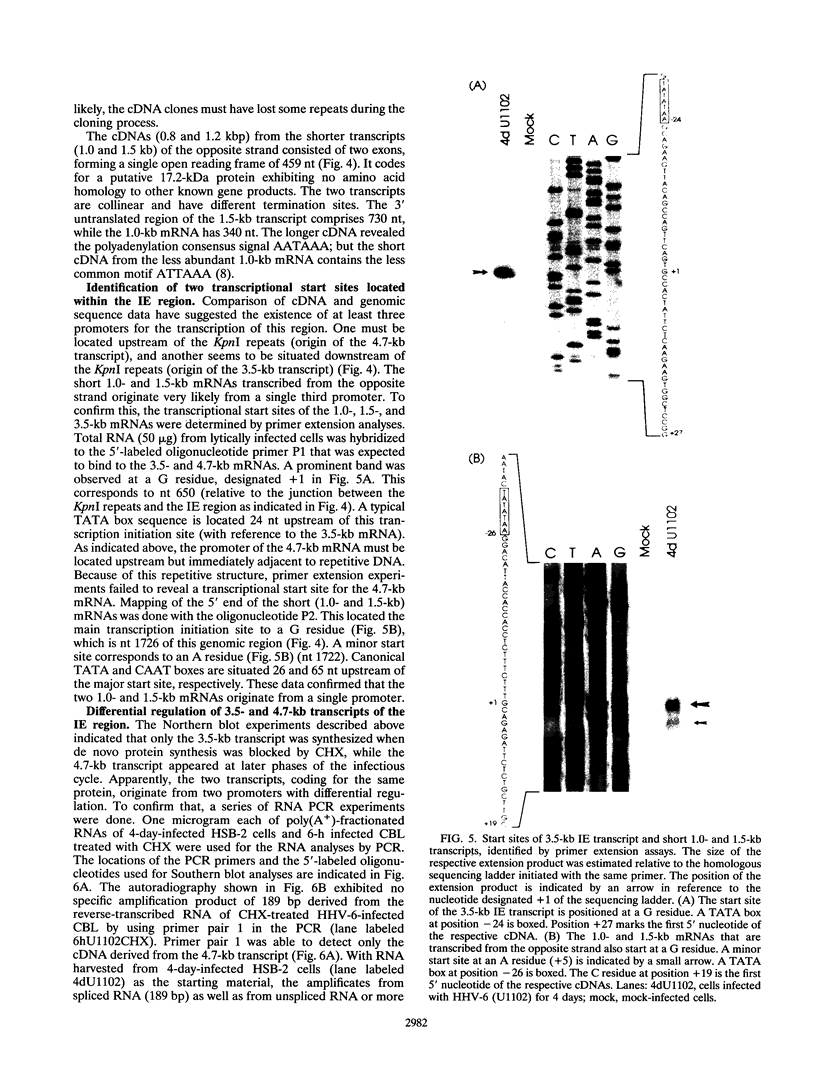
The unique segment of the human herpesvirus 6 (HHV-6) genome is essentially collinear to the unique long DNA segment of another betaherpesvirus, the human cytomegalovirus (HCMV). However, the HHV-6 genomic section that is analogous in position to the major immediate-early (IE) locus of HCMV does not exhibit recognizable sequence homologies. The respective HHV-6 region of 5.5 kbp is flanked on one side by 25 to 28 incomplete tandem repeats of 105 to 110 bp that contain, with one exception, a single KpnI restriction site (KpnI repeats). About 250 reiterations of the sequence motif CACATA are located on the other end. We identified two open reading frames of 375 and 2,595 nucleotides, respectively, on one strand. Strand-specific Northern blot analyses with RNA harvested from HHV-6 (strain U1102)-infected HSB-2 cells or cord blood lymphocytes revealed two transcripts of about 3.5 and 4.7 kb in the corresponding orientation. Sequence analyses of the respective cDNA clones and primer extension experiments were used to map the mRNAs. The two transcripts are coterminal and multiply spliced and code for the same putative 104.6-kDa protein, but they are initiated from different promoters. Characterization of smaller cDNA clones and Northern blotting with other strand-specific probes showed that singly spliced mRNAs of 1.0 and 1.5 kb are transcribed from the opposite strand; they could code for a 17.2-kDa polypeptide. Blocking experiments with cycloheximide led to the conclusion that only the 3.5-kb mRNA is synthesized in the absence of protein biosynthesis upon infection with cell-free virus. This identifies a single IE gene of HHV-6 at the genomic position corresponding to the major IE region of HCMV, although the coding content and transcriptional regulation are quite different for these two herpesvirus IE regions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Salahuddin S. Z., Josephs S. F., Imam F., Lusso P., Gallo R. C., Hung C., Lemp J., Markham P. D. HBLV (or HHV-6) in human cell lines. Nature. 1987 Sep 17;329(6136):207–207. doi: 10.1038/329207a0. [DOI] [PubMed] [Google Scholar]
- Akrigg A., Wilkinson G. W., Oram J. D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985 Mar;2(2):107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- Albrecht J. C., Fleckenstein B. Structural organization of the conserved gene block of Herpesvirus saimiri coding for DNA polymerase, glycoprotein B, and major DNA binding protein. Virology. 1990 Feb;174(2):533–542. doi: 10.1016/0042-6822(90)90107-3. [DOI] [PubMed] [Google Scholar]
- Asano Y., Yoshikawa T., Suga S., Nakashima T., Yazaki T., Fukuda M., Kojima S., Matsuyama T. Reactivation of herpesvirus type 6 in children receiving bone marrow transplants for leukemia. N Engl J Med. 1991 Feb 28;324(9):634–635. doi: 10.1056/NEJM199102283240915. [DOI] [PubMed] [Google Scholar]
- Biegalke B. J., Geballe A. P. Sequence requirements for activation of the HIV-1 LTR by human cytomegalovirus. Virology. 1991 Jul;183(1):381–385. doi: 10.1016/0042-6822(91)90151-z. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Borisch B., Ellinger K., Neipel F., Fleckenstein B., Kirchner T., Ott M. M., Müller-Hermelink H. K. Lymphadenitis and lymphoproliferative lesions associated with the human herpes virus-6 (HHV-6). Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61(3):179–187. doi: 10.1007/BF02890420. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Drobyski W. R., Russler S. K., Tapper M. A., Knox K. K., Ash R. C. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991 Jul 20;338(8760):147–149. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Keil G. M., Weber F., Jasin M., Schaffner W., Koszinowski U. H. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyski W. R., Dunne W. M., Burd E. M., Knox K. K., Ash R. C., Horowitz M. M., Flomenberg N., Carrigan D. R. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipients: evidence of a marrow-suppressive role for HHV-6 in vivo. J Infect Dis. 1993 Mar;167(3):735–739. doi: 10.1093/infdis/167.3.735. [DOI] [PubMed] [Google Scholar]
- Ellinger K., Neipel F., Foà-Tomasi L., Campadelli-Fiume G., Fleckenstein B. The glycoprotein B homologue of human herpesvirus 6. J Gen Virol. 1993 Mar;74(Pt 3):495–500. doi: 10.1099/0022-1317-74-3-495. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Young J., Giulietti E., DeMattei C., Garcia J., Gaynor R., Stenberg R. M., Nelson J. A. A discrete cis element in the human immunodeficiency virus long terminal repeat mediates synergistic trans activation by cytomegalovirus immediate-early proteins. J Virol. 1991 Dec;65(12):6735–6742. doi: 10.1128/jvi.65.12.6735-6742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier C., Walker S., Caswell R., Kouzarides T., Sinclair J. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J Virol. 1992 Jul;66(7):4452–4456. doi: 10.1128/jvi.66.7.4452-4456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. B. Herpes and the rash of roses: a new virus, HHV-6, as a cause of an old childhood disease, roseola. Pediatr Ann. 1990 Sep;19(9):517–521. doi: 10.3928/0090-4481-19900901-07. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Gompels U. A., Barrell B. G., Craxton M., Cameron K. R., Staden R., Chang Y. N., Hayward G. S. Deviations from expected frequencies of CpG dinucleotides in herpesvirus DNAs may be diagnostic of differences in the states of their latent genomes. J Gen Virol. 1989 Apr;70(Pt 4):837–855. doi: 10.1099/0022-1317-70-4-837. [DOI] [PubMed] [Google Scholar]
- Human herpesvirus-6 strain groups: a nomenclature. Arch Virol. 1993;129(1-4):363–366. doi: 10.1007/BF01316913. [DOI] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Cho M. S., Hayward G. S. Abundant constitutive expression of the immediate-early 94K protein from cytomegalovirus (Colburn) in a DNA-transfected mouse cell line. Mol Cell Biol. 1984 Oct;4(10):2214–2223. doi: 10.1128/mcb.4.10.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger G. R., Manak M., Bourgeois N., Ablashi D. V., Salahuddin S. Z., Josephs S. S., Buchbinder A., Gallo R. C., Berthold F., Tesch H. Persistent active herpes virus infection associated with atypical polyclonal lymphoproliferation (APL) and malignant lymphoma. Anticancer Res. 1989 Nov-Dec;9(6):1457–1476. [PubMed] [Google Scholar]
- Larcher C., Huemer H. P., Margreiter R., Dierich M. P. Serological crossreaction of human herpesvirus-6 with cytomegalovirus. Lancet. 1988 Oct 22;2(8617):963–964. doi: 10.1016/s0140-6736(88)92631-1. [DOI] [PubMed] [Google Scholar]
- Lau R., Packham G., Farrell P. J. Differential splicing of Epstein-Barr virus immediate-early RNA. J Virol. 1992 Oct;66(10):6233–6236. doi: 10.1128/jvi.66.10.6233-6236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Landay A., Lennette E. T. Human herpesvirus 6 inhibits human immunodeficiency virus type 1 replication in cell culture. J Clin Microbiol. 1990 Oct;28(10):2362–2364. doi: 10.1128/jcm.28.10.2362-2364.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquester G. J., Pellett P. E. Properties of the human herpesvirus 6 strain Z29 genome: G + C content, length, and presence of variable-length directly repeated terminal sequence elements. Virology. 1991 May;182(1):102–110. doi: 10.1016/0042-6822(91)90653-s. [DOI] [PubMed] [Google Scholar]
- Lopez C., Pellett P., Stewart J., Goldsmith C., Sanderlin K., Black J., Warfield D., Feorino P. Characteristics of human herpesvirus-6. J Infect Dis. 1988 Jun;157(6):1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- Manet E., Gruffat H., Trescol-Biemont M. C., Moreno N., Chambard P., Giot J. F., Sergeant A. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989 Jun;8(6):1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis D. M., Rabson A. B., Straus S. E., Ostrove J. M. Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology. 1992 Feb;186(2):788–791. doi: 10.1016/0042-6822(92)90048-t. [DOI] [PubMed] [Google Scholar]
- Martin M. E., Nicholas J., Thomson B. J., Newman C., Honess R. W. Identification of a transactivating function mapping to the putative immediate-early locus of human herpesvirus 6. J Virol. 1991 Oct;65(10):5381–5390. doi: 10.1128/jvi.65.10.5381-5390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neipel F., Ellinger K., Fleckenstein B. Gene for the major antigenic structural protein (p100) of human herpesvirus 6. J Virol. 1992 Jun;66(6):3918–3924. doi: 10.1128/jvi.66.6.3918-3924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neipel F., Ellinger K., Fleckenstein B. The unique region of the human herpesvirus 6 genome is essentially collinear with the UL segment of human cytomegalovirus. J Gen Virol. 1991 Sep;72(Pt 9):2293–2297. doi: 10.1099/0022-1317-72-9-2293. [DOI] [PubMed] [Google Scholar]
- Perera L. P., Mosca J. D., Ruyechan W. T., Hayward G. S., Straus S. E., Hay J. A major transactivator of varicella-zoster virus, the immediate-early protein IE62, contains a potent N-terminal activation domain. J Virol. 1993 Aug;67(8):4474–4483. doi: 10.1128/jvi.67.8.4474-4483.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietroboni G. R., Harnett G. B., Bucens M. R. Centrifugal enhancement of human immunodeficiency virus (HIV) and human herpesvirus type 6 (HHV-6) infection in vitro. J Virol Methods. 1989 Apr-May;24(1-2):85–90. doi: 10.1016/0166-0934(89)90010-4. [DOI] [PubMed] [Google Scholar]
- Puchtler E., Stamminger T. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J Virol. 1991 Nov;65(11):6301–6306. doi: 10.1128/jvi.65.11.6301-6306.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson W. D., Barrell B. G. Spliced transcripts of human cytomegalovirus. J Virol. 1993 Sep;67(9):5502–5513. doi: 10.1128/jvi.67.9.5502-5513.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizmann B., Desrosiers R. C., Fleckenstein B., Lopez C., Minson A. C., Studdert M. J. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch Virol. 1992;123(3-4):425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Stamminger T., Fleckenstein B. Immediate-early transcription regulation of human cytomegalovirus. Curr Top Microbiol Immunol. 1990;154:3–19. doi: 10.1007/978-3-642-74980-3_1. [DOI] [PubMed] [Google Scholar]
- Stenberg R. M., Depto A. S., Fortney J., Nelson J. A. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J Virol. 1989 Jun;63(6):2699–2708. doi: 10.1128/jvi.63.6.2699-2708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Witte P. R., Stinski M. F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985 Dec;56(3):665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzl M., Roth W. K. "Run-off" synthesis and application of defined single-stranded DNA hybridization probes. Anal Biochem. 1990 Feb 15;185(1):164–169. doi: 10.1016/0003-2697(90)90274-d. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J., Spector D. J., Leisure K. M., Stinski M. F. Participation of two human cytomegalovirus immediate early gene regions in transcriptional activation of adenovirus promoters. Virology. 1987 Dec;161(2):276–285. doi: 10.1016/0042-6822(87)90119-x. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson B. J., Honess R. W. The right end of the unique region of the genome of human herpesvirus 6 U1102 contains a candidate immediate early gene enhancer and a homologue of the human cytomegalovirus US22 gene family. J Gen Virol. 1992 Jul;73(Pt 7):1649–1660. doi: 10.1099/0022-1317-73-7-1649. [DOI] [PubMed] [Google Scholar]
- Wirth U. V., Fraefel C., Vogt B., Vlcek C., Paces V., Schwyzer M. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3' coterminal and encode a putative zinc finger transactivator protein. J Virol. 1992 May;66(5):2763–2772. doi: 10.1128/jvi.66.5.2763-2772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yeung K. C., Stoltzfus C. M., Stinski M. F. Mutations of the human cytomegalovirus immediate-early 2 protein defines regions and amino acid motifs important in transactivation of transcription from the HIV-1 LTR promoter. Virology. 1993 Aug;195(2):786–792. doi: 10.1006/viro.1993.1431. [DOI] [PubMed] [Google Scholar]
- Yoshiyama H., Suzuki E., Yoshida T., Kajii T., Yamamoto N. Role of human herpesvirus 6 infection in infants with exanthema subitum. Pediatr Infect Dis J. 1990 Feb;9(2):71–74. doi: 10.1097/00006454-199002000-00001. [DOI] [PubMed] [Google Scholar]