Abstract
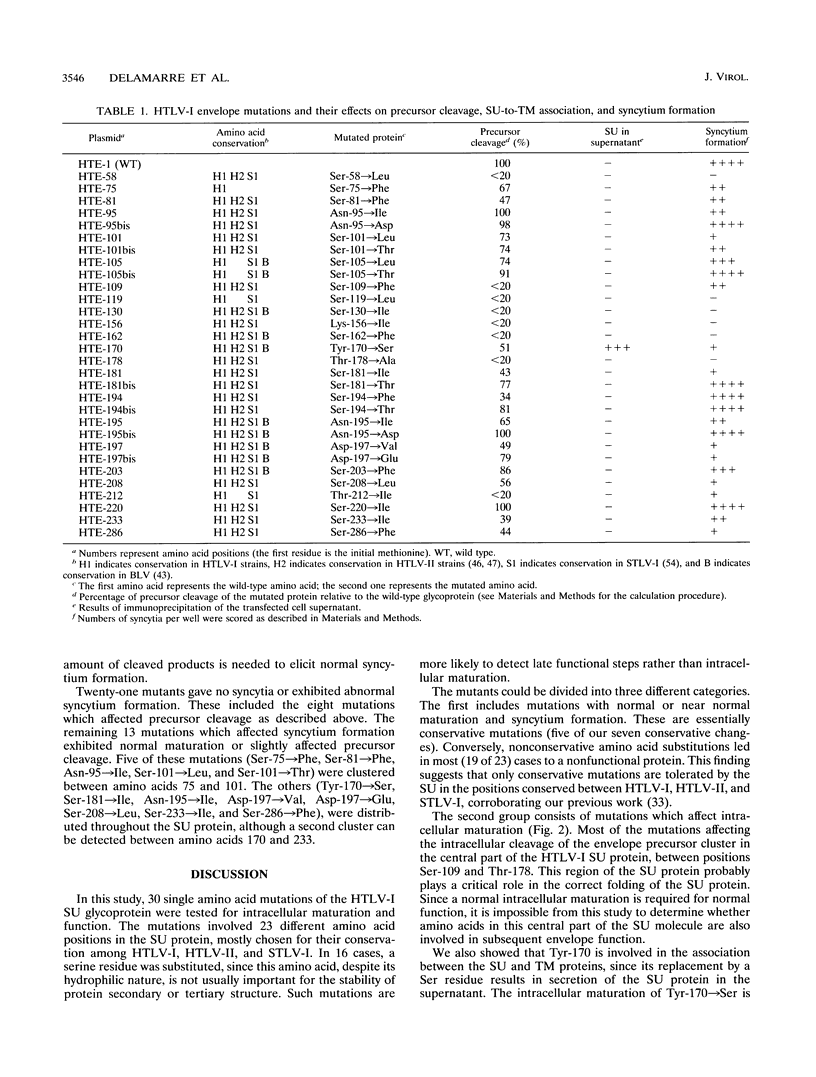
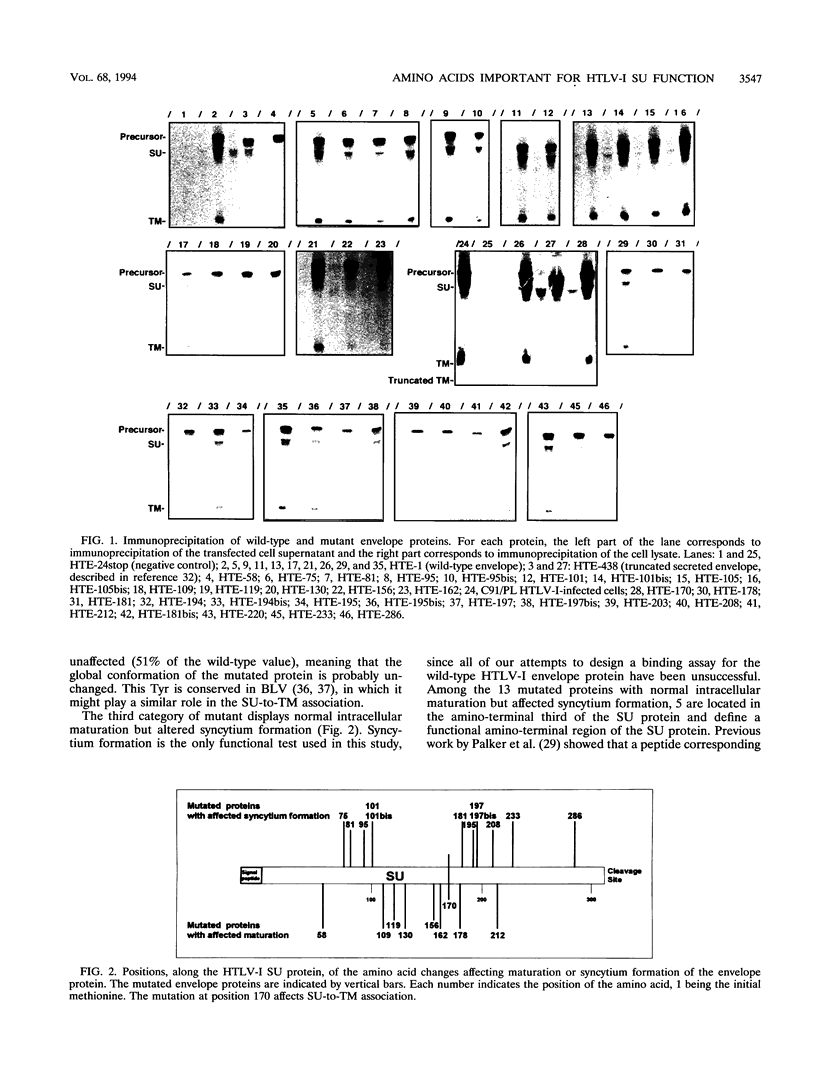
Single conservative and nonconservative amino acid substitutions were introduced into the gp45 external envelope protein (SU) of human T-cell leukemia virus type I (HTLV-I). The mutated amino acids were those identified as being conserved in HTLV-I, HTLV-II, and simian T-cell leukemia virus type I (but not in bovine leukemia virus). The mutated envelopes were tested for intracellular maturation and for function. Mutants with three major phenotypes could be defined: (i) 9 mutants with a wild-type phenotype, which included most of the conservative amino acid changes (five of seven) distributed throughout the SU protein; (ii) 8 mutants with affected intracellular maturation, 6 of which define a region in the central part of the SU protein essential for correct folding of the protein; and (iii) 13 mutants with normal intracellular maturation but impaired syncytium formation. These mutations likely affect the receptor binding step or postbinding events required for fusion. Five of these mutations are located between amino acids 75 and 101 of the SU protein, in the amino-terminal third of the molecule. The other mutations involve positions 170, 181, 195, 197, 208, 233, and 286, suggesting that two other domains, one central and one carboxy terminal, are involved in HTLV-I envelope functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba E., Nakamura M., Tanaka Y., Kuroki M., Itoyama Y., Nakano S., Niho Y. Multiple neutralizing B-cell epitopes of human T-cell leukemia virus type 1 (HTLV-1) identified by human monoclonal antibodies. A basis for the design of an HTLV-1 peptide vaccine. J Immunol. 1993 Jul 15;151(2):1013–1024. [PubMed] [Google Scholar]
- Battini J. L., Heard J. M., Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992 Mar;66(3):1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Lee T. H., Samuel K. P., Okayama A., Tachibana N., Miyoshi I., Papas T. S., Essex M. Antibody reactivity to different regions of human T-cell leukemia virus type 1 gp61 in infected people. J Virol. 1989 Nov;63(11):4952–4957. doi: 10.1128/jvi.63.11.4952-4957.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Daenke S., Nightingale S., Cruickshank J. K., Bangham C. R. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J Virol. 1990 Mar;64(3):1278–1282. doi: 10.1128/jvi.64.3.1278-1282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. K., Lairmore M. D., Griffis K., Williams L. J., Villinger F., Quinn T. C., Brown C., Nzilambi, Sugimoto M., Araki S. Comparative analysis of nucleotide sequences of the partial envelope gene (5' domain) among human T lymphotropic virus type I (HTLV-I) isolates. Virology. 1991 May;182(1):413–419. doi: 10.1016/0042-6822(91)90692-5. [DOI] [PubMed] [Google Scholar]
- Dokhelar M. C., Pickford H., Sodroski J., Haseltine W. A. HTLV-I p27rex regulates gag and env protein expression. J Acquir Immune Defic Syndr. 1989;2(5):431–440. [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Fung M. S., Sun C. R., Gordon W. L., Liou R. S., Chang T. W., Sun W. N., Daar E. S., Ho D. D. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J Virol. 1992 Feb;66(2):848–856. doi: 10.1128/jvi.66.2.848-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gray G. S., White M., Bartman T., Mann D. Envelope gene sequence of HTLV-1 isolate MT-2 and its comparison with other HTLV-1 isolates. Virology. 1990 Jul;177(1):391–395. doi: 10.1016/0042-6822(90)90498-g. [DOI] [PubMed] [Google Scholar]
- Hattori S., Kiyokawa T., Imagawa K., Shimizu F., Hashimura E., Seiki M., Yoshida M. Identification of gag and env gene products of human T-cell leukemia virus (HTLV). Virology. 1984 Jul 30;136(2):338–347. doi: 10.1016/0042-6822(84)90170-3. [DOI] [PubMed] [Google Scholar]
- Horal P., Hall W. W., Svennerholm B., Lycke J., Jeansson S., Rymo L., Kaplan M. H., Vahlne A. Identification of type-specific linear epitopes in the glycoproteins gp46 and gp21 of human T-cell leukemia viruses type I and type II using synthetic peptides. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5754–5758. doi: 10.1073/pnas.88.13.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Tsujimoto A., Shimotohno K. Sequence variations in LTR and env regions of HTLV-I do not discriminate between the virus from patients with HTLV-I-associated myelopathy and adult T-cell leukemia. Int J Cancer. 1991 Feb 20;47(4):491–495. doi: 10.1002/ijc.2910470403. [DOI] [PubMed] [Google Scholar]
- Komurian F., Pelloquin F., de Thé G. In vivo genomic variability of human T-cell leukemia virus type I depends more upon geography than upon pathologies. J Virol. 1991 Jul;65(7):3770–3778. doi: 10.1128/jvi.65.7.3770-3778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuroda N., Washitani Y., Shiraki H., Kiyokawa H., Ohno M., Sato H., Maeda Y. Detection of antibodies to human T-lymphotropic virus type I by using synthetic peptides. Int J Cancer. 1990 May 15;45(5):865–868. doi: 10.1002/ijc.2910450514. [DOI] [PubMed] [Google Scholar]
- Kuroki M., Nakamura M., Itoyama Y., Tanaka Y., Shiraki H., Baba E., Esaki T., Tatsumoto T., Nagafuchi S., Nakano S. Identification of new epitopes recognized by human monoclonal antibodies with neutralizing and antibody-dependent cellular cytotoxicity activities specific for human T cell leukemia virus type 1. J Immunol. 1992 Aug 1;149(3):940–948. [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Homma T., McLane M. F., Tachibana N., Essex M. Human T-cell leukemia virus-associated membrane antigens: identity of the major antigens recognized after virus infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3856–3860. doi: 10.1073/pnas.81.12.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. T., Even J., Karpas A. Molecular cloning and complete nucleotide sequence of an adult T cell leukaemia virus/human T cell leukaemia virus type I (ATLV/HTLV-I) isolate of Caribbean origin: relationship to other members of the ATLV/HTLV-I subgroup. J Gen Virol. 1988 Jul;69(Pt 7):1695–1710. doi: 10.1099/0022-1317-69-7-1695. [DOI] [PubMed] [Google Scholar]
- Murphy E. L., Hanchard B., Figueroa J. P., Gibbs W. N., Lofters W. S., Campbell M., Goedert J. J., Blattner W. A. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989 Feb 15;43(2):250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- Ott D., Friedrich R., Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990 Feb;64(2):757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott D., Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992 Aug;66(8):4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine E., Garcia J., Philpott T. C., Shaw G., Ratner L. Limited sequence variation in human T-lymphotropic virus type 1 isolates from North American and African patients. Virology. 1991 May;182(1):111–123. doi: 10.1016/0042-6822(91)90654-t. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Riggs E. R., Spragion D. E., Muir A. J., Scearce R. M., Randall R. R., McAdams M. W., McKnight A., Clapham P. R., Weiss R. A. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J Virol. 1992 Oct;66(10):5879–5889. doi: 10.1128/jvi.66.10.5879-5889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Tanner M. E., Scearce R. M., Streilein R. D., Clark M. E., Haynes B. F. Mapping of immunogenic regions of human T cell leukemia virus type I (HTLV-I) gp46 and gp21 envelope glycoproteins with env-encoded synthetic peptides and a monoclonal antibody to gp46. J Immunol. 1989 Feb 1;142(3):971–978. [PubMed] [Google Scholar]
- Pique C., Pham D., Tursz T., Dokhélar M. C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992 Feb;66(2):906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique C., Pham D., Tursz T., Dokhélar M. C. The cytoplasmic domain of the human T-cell leukemia virus type I envelope can modulate envelope functions in a cell type-dependent manner. J Virol. 1993 Jan;67(1):557–561. doi: 10.1128/jvi.67.1.557-561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique C., Tursz T., Dokhelar M. C. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: a basis for envelope conservation? EMBO J. 1990 Dec;9(13):4243–4248. doi: 10.1002/j.1460-2075.1990.tb07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S. R., Rosa M. D., Rosa J. J., Wiley D. C. Truncated variants of gp120 bind CD4 with high affinity and suggest a minimum CD4 binding region. EMBO J. 1992 Feb;11(2):585–591. doi: 10.1002/j.1460-2075.1992.tb05090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portetelle D., Couez D., Bruck C., Kettmann R., Mammerickx M., Van der Maaten M., Brasseur R., Burny A. Antigenic variants of bovine leukemia virus (BLV) are defined by amino acid substitutions in the NH2 part of the envelope glycoprotein gp51. Virology. 1989 Mar;169(1):27–33. doi: 10.1016/0042-6822(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Couez D., Deschamps J., Kettmann R., Burny A., Gilden R. V. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology. 1984 Oct 15;138(1):82–93. doi: 10.1016/0042-6822(84)90149-1. [DOI] [PubMed] [Google Scholar]
- SVOBODA J., CHYLE P., SIMKOVIC D., HILGERT I. Demonstration of the absence of infectious Rous virus in rat tumour XC, whose structurally intact cells produce Rous sarcoma when transferred to chicks. Folia Biol (Praha) 1963 Apr;9:77–81. [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxinger W., Blattner W. A., Levine P. H., Clark J., Biggar R., Hoh M., Moghissi J., Jacobs P., Wilson L., Jacobson R. Human T-cell leukemia virus (HTLV-I) antibodies in Africa. Science. 1984 Sep 28;225(4669):1473–1476. doi: 10.1126/science.6089348. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Calabrò M. L., Hoad J. G., Carrington C. V., Matutes E., Catovsky D., Weiss R. A. HTLV-1 envelope sequences from Brazil, the Caribbean, and Romania: clustering of sequences according to geographic origin and variability in an antibody epitope. Virology. 1991 Oct;184(2):483–491. doi: 10.1016/0042-6822(91)90418-b. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D. W., Chen I. S., Miwa M., Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Perkins D., Briggs D., Lee T. H., Essex M., Coligan J., Wong-Staal F., Gallo R. C., Haseltine W. A. Sequence of the envelope glycoprotein gene of type II human T lymphotropic virus. Science. 1984 Jul 27;225(4660):421–424. doi: 10.1126/science.6204380. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987 Sep;61(9):2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N., Thali M., Furman C., Ho D. D., Sodroski J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J Virol. 1993 Jun;67(6):3674–3679. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Zeng L., Shiraki H., Shida H., Tozawa H. Identification of a neutralization epitope on the envelope gp46 antigen of human T cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J Immunol. 1991 Jul 1;147(1):354–360. [PubMed] [Google Scholar]
- Tsujimoto A., Teruuchi T., Imamura J., Shimotohno K., Miyoshi I., Miwa M. Nucleotide sequence analysis of a provirus derived from HTLV-1-associated myelopathy (HAM). Mol Biol Med. 1988 Feb;5(1):29–42. [PubMed] [Google Scholar]
- Watanabe T., Seiki M., Tsujimoto H., Miyoshi I., Hayami M., Yoshida M. Sequence homology of the simian retrovirus genome with human T-cell leukemia virus type I. Virology. 1985 Jul 15;144(1):59–65. doi: 10.1016/0042-6822(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Wyatt R., Thali M., Tilley S., Pinter A., Posner M., Ho D., Robinson J., Sodroski J. Relationship of the human immunodeficiency virus type 1 gp120 third variable loop to a component of the CD4 binding site in the fourth conserved region. J Virol. 1992 Dec;66(12):6997–7004. doi: 10.1128/jvi.66.12.6997-7004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



