Abstract
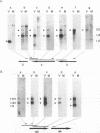
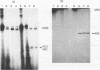
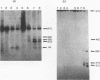
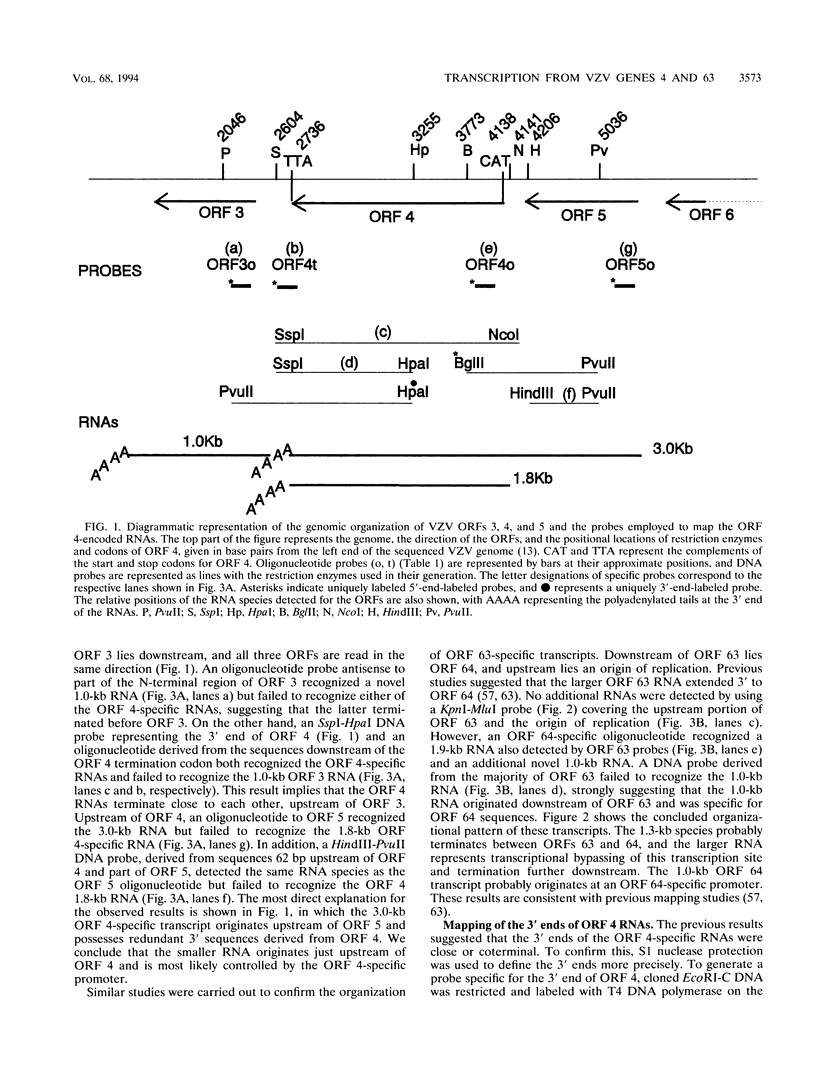
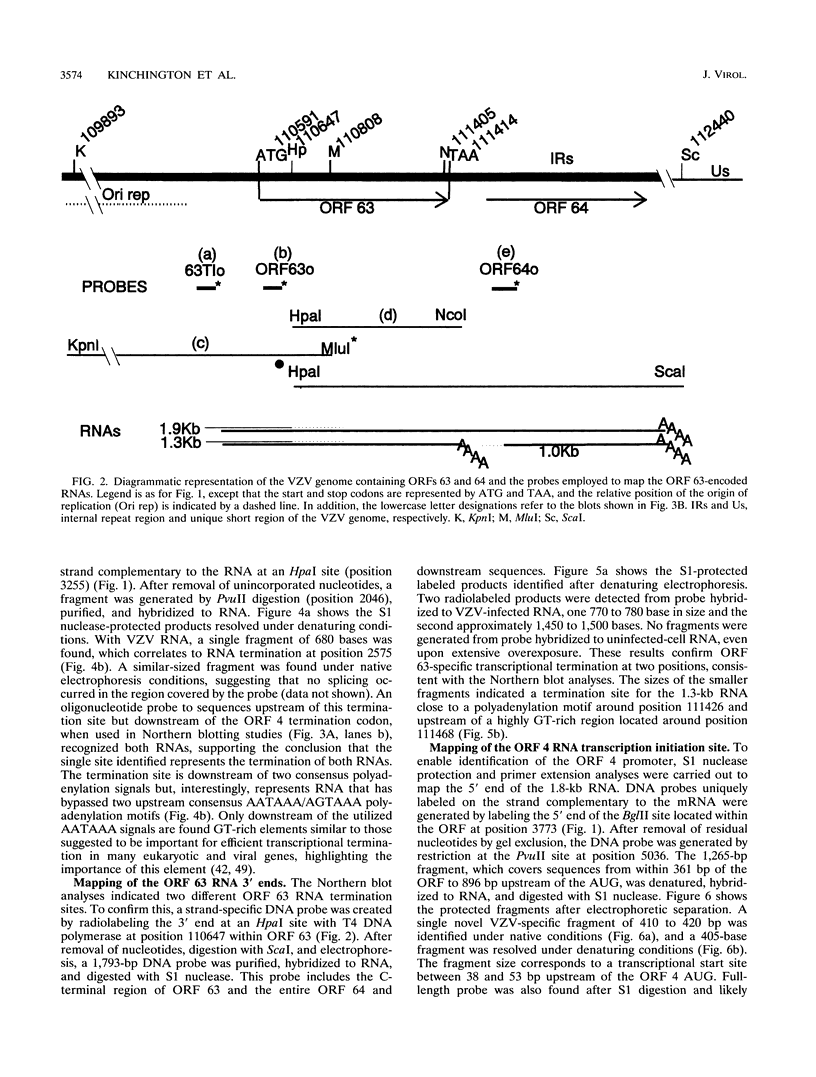
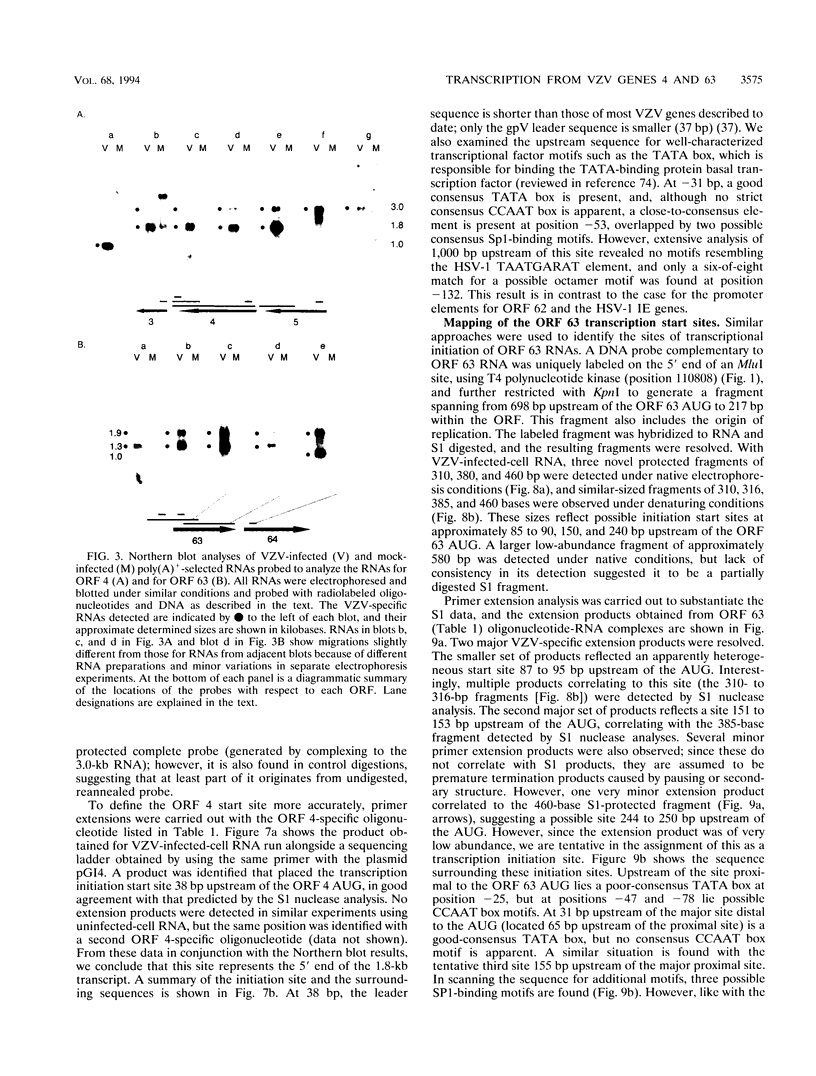
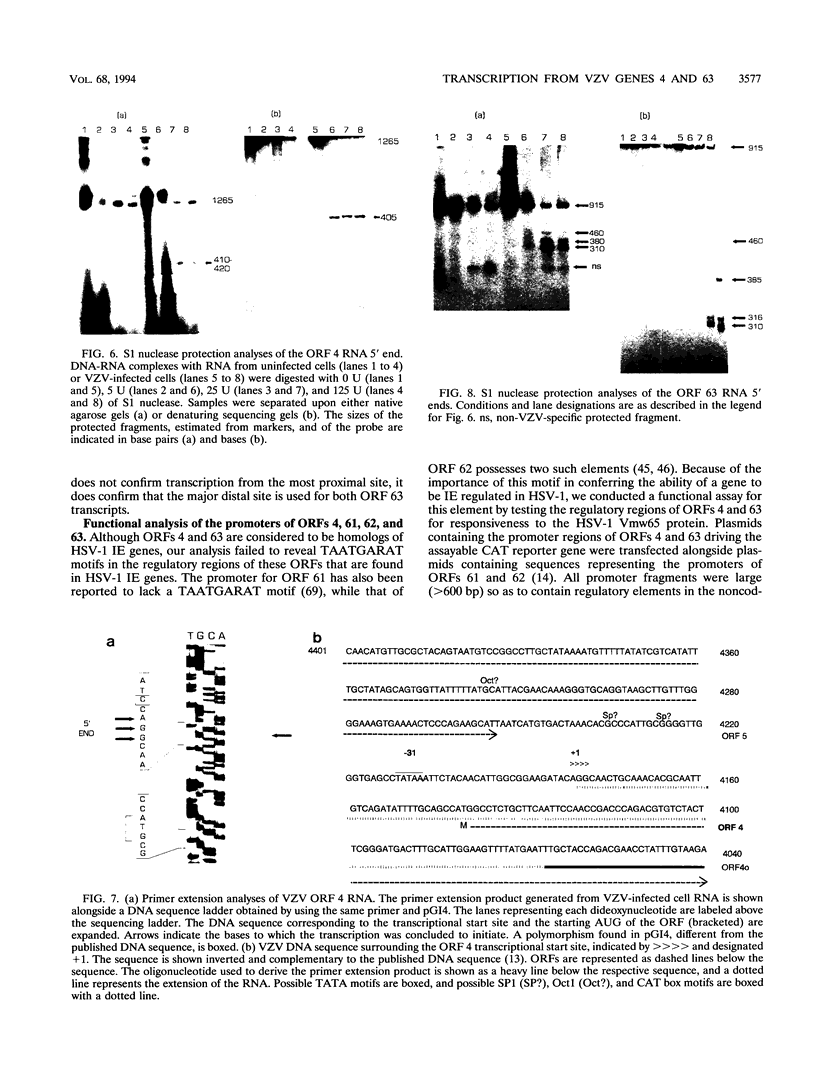
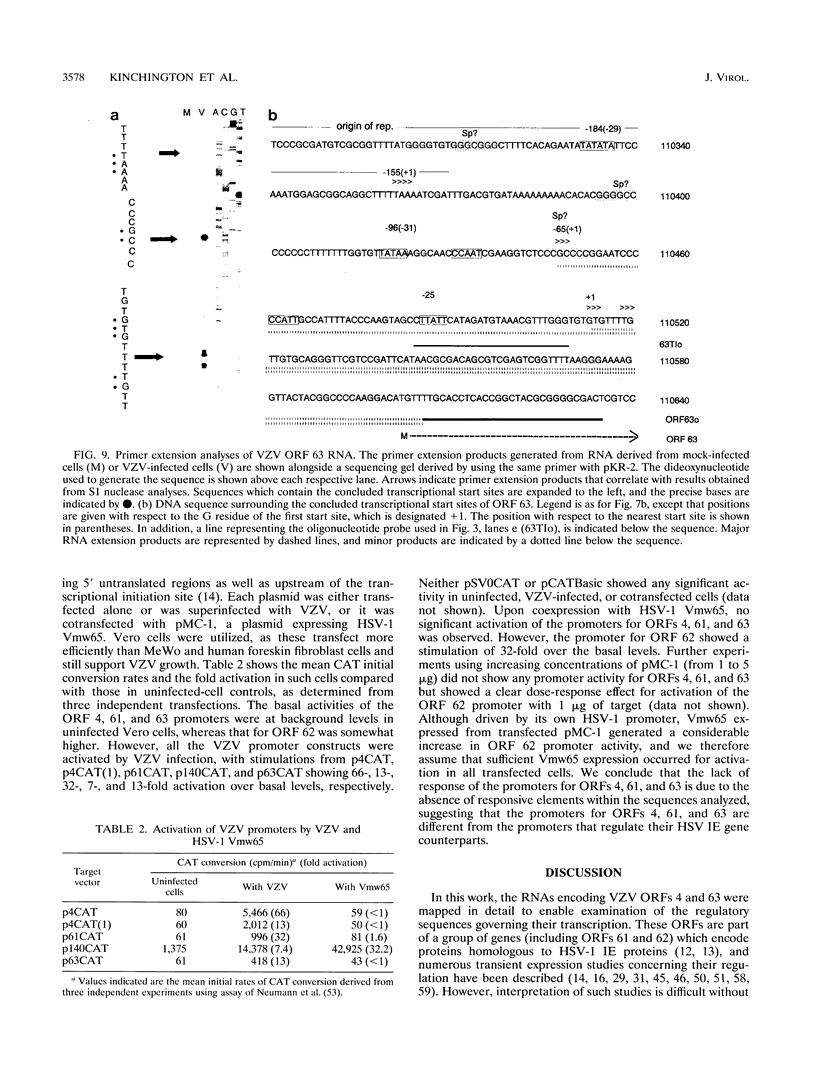
Four of the 68 varicella-zoster virus (VZV) unique open reading frames (ORFs), i.e., ORFs 4, 61, 62, and 63, encode proteins that influence viral transcription and are considered to be positional homologs of herpes simplex virus type 1 (HSV-1) immediate-early (IE) proteins. In order to identify the elements that regulate transcription of VZV ORFs 4 and 63, the encoded mRNAs were mapped in detail. For ORF 4, a major 1.8-kb and a minor 3.0-kb polyadenylated [poly(A)+] RNA were identified, whereas ORF 63-specific probes recognized 1.3- and 1.9-kb poly(A)+ RNAs. Probes specific for sequences adjacent to the ORFs and mapping of the RNA 3' ends indicated that the ORF 4 RNAs were 3' coterminal, whereas the RNAs for ORF 63 represented two different termination sites. S1 nuclease mapping and primer extension analyses indicated a single transcription initiation site for ORF 4 at 38 bp upstream of the ORF start codon. For ORF 63, multiple transcriptional start sites at 87 to 95, 151 to 153, and (tentatively) 238 to 243 bp upstream of the ORF start codon were identified. TATA box motifs at good positional locations were found upstream of all mapped transcription initiation sites. However, no sequences resembling the TAATGARAT motif, which confers IE regulation upon HSV-1 IE genes, were found. The finding of the absence of this motif was supported through analyses of the regulatory sequences of ORFs 4 and 63 in transient transfection assays alongside those of ORFs 61 and 62. Sequences representing the promoters for ORFs 4, 61, and 63 were all stimulated by VZV infection but failed to be stimulated by coexpression with the HSV-1 transactivator Vmw65. In contrast, the promoter for ORF 62, which contains TAATGARAT motifs, was activated by VZV infection and coexpression with Vmw65. These results extend the transcriptional knowledge for VZV and suggest that ORFs 4 and 63 contain regulatory signals different from those of the ORF 62 and HSV-1 IE genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ace C. I., Dalrymple M. A., Ramsay F. H., Preston V. G., Preston C. M. Mutational analysis of the herpes simplex virus type 1 trans-inducing factor Vmw65. J Gen Virol. 1988 Oct;69(Pt 10):2595–2605. doi: 10.1099/0022-1317-69-10-2595. [DOI] [PubMed] [Google Scholar]
- Ace C. I., McKee T. A., Ryan J. M., Cameron J. M., Preston C. M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989 May;63(5):2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson D. W., Leen C. L., Tariq W. U., Mandal B. K. Severe and recurrent varicella-zoster virus infection in a patient with the acquired immune deficiency syndrome. J Infect. 1988 Mar;16(2):193–197. doi: 10.1016/s0163-4453(88)94122-9. [DOI] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabirac G. F., Mahalingam R., Wellish M., Gilden D. H. Trans-activation of viral tk promoters by proteins encoded by varicella zoster virus open reading frames 61 and 62. Virus Res. 1990 Jan;15(1):57–68. doi: 10.1016/0168-1702(90)90013-2. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Preston C. M. DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology. 1987 Apr;157(2):307–316. doi: 10.1016/0042-6822(87)90273-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cousens D. J., Greaves R., Goding C. R., O'Hare P. The C-terminal 79 amino acids of the herpes simplex virus regulatory protein, Vmw65, efficiently activate transcription in yeast and mammalian cells in chimeric DNA-binding proteins. EMBO J. 1989 Aug;8(8):2337–2342. doi: 10.1002/j.1460-2075.1989.tb08361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Varicella-zoster virus. The Fourteenth Fleming lecture. J Gen Virol. 1991 Mar;72(Pt 3):475–486. doi: 10.1099/0022-1317-72-3-475. [DOI] [PubMed] [Google Scholar]
- Defechereux P., Melen L., Baudoux L., Merville-Louis M. P., Rentier B., Piette J. Characterization of the regulatory functions of varicella-zoster virus open reading frame 4 gene product. J Virol. 1993 Jul;67(7):4379–4385. doi: 10.1128/jvi.67.7.4379-4385.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney G. H., Everett R. D. A herpes simplex virus type 1 recombinant with both copies of the Vmw175 coding sequences replaced by the homologous varicella-zoster virus open reading frame. J Gen Virol. 1990 Nov;71(Pt 11):2681–2689. doi: 10.1099/0022-1317-71-11-2681. [DOI] [PubMed] [Google Scholar]
- Disney G. H., McKee T. A., Preston C. M., Everett R. D. The product of varicella-zoster virus gene 62 autoregulates its own promoter. J Gen Virol. 1990 Dec;71(Pt 12):2999–3003. doi: 10.1099/0022-1317-71-12-2999. [DOI] [PubMed] [Google Scholar]
- Everett R. D. The regulation of transcription of viral and cellular genes by herpesvirus immediate-early gene products (review). Anticancer Res. 1987 Jul-Aug;7(4A):589–604. [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Kinchington P. R., Inchauspe G., Straus S. E., Ostrove J. M. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the "IE" 175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988 Jun;62(6):2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Straus S. E., Ostrove J. M. Varicella-zoster virus complements herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1987 Jan;61(1):225–228. doi: 10.1128/jvi.61.1.225-228.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Mahalingam R., Vafai A., Hurst J. W., Dupuis K. W. Monoclonal antibody to immediate early protein encoded by varicella-zoster virus gene 62. Virus Res. 1990 Jun;16(2):195–210. doi: 10.1016/0168-1702(90)90023-5. [DOI] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., O'Callaghan D. J., Staczek J. Characterization and mapping of equine herpesvirus type 1 immediate early, early, and late transcripts. Virus Res. 1987 Sep;8(3):233–244. doi: 10.1016/0168-1702(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe G., Nagpal S., Ostrove J. M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989 Dec;173(2):700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Inchauspe G., Ostrove J. M. Differential regulation by varicella-zoster virus (VZV) and herpes simplex virus type-1 trans-activating genes. Virology. 1989 Dec;173(2):710–714. doi: 10.1016/0042-6822(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Jackers P., Defechereux P., Baudoux L., Lambert C., Massaer M., Merville-Louis M. P., Rentier B., Piette J. Characterization of regulatory functions of the varicella-zoster virus gene 63-encoded protein. J Virol. 1992 Jun;66(6):3899–3903. doi: 10.1128/jvi.66.6.3899-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Kitamura K., Hayakawa Y., Takahashi M., Kojima A., Sato S., Yamanishi K. Transcription mapping of glycoprotein I (gpI) and gpIV of varicella-zoster virus and immunological analysis of the gpI produced in cells infected with the recombinant vaccinia virus. Microbiol Immunol. 1989;33(4):299–312. doi: 10.1111/j.1348-0421.1989.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Kinchington P. R., Hougland J. K., Arvin A. M., Ruyechan W. T., Hay J. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J Virol. 1992 Jan;66(1):359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington P. R., Inchauspe G., Subak-Sharpe J. H., Robey F., Hay J., Ruyechan W. T. Identification and characterization of a varicella-zoster virus DNA-binding protein by using antisera directed against a predicted synthetic oligopeptide. J Virol. 1988 Mar;62(3):802–809. doi: 10.1128/jvi.62.3.802-809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington P. R., Remenick J., Ostrove J. M., Straus S. E., Ruyechan W. T., Hay J. Putative glycoprotein gene of varicella-zoster virus with variable copy numbers of a 42-base-pair repeat sequence has homology to herpes simplex virus glycoprotein C. J Virol. 1986 Sep;59(3):660–668. doi: 10.1128/jvi.59.3.660-668.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T. M., Roizman B. Host cell proteins bind to the cis-acting site required for virion-mediated induction of herpes simplex virus 1 alpha genes. Proc Natl Acad Sci U S A. 1987 Jan;84(1):71–75. doi: 10.1073/pnas.84.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P., Kinchington P. R., Ruyechan W. T., Hay J. A detailed analysis of transcripts mapping to varicella zoster virus gene 14 (glycoprotein V). Virology. 1991 Oct;184(2):625–635. doi: 10.1016/0042-6822(91)90432-b. [DOI] [PubMed] [Google Scholar]
- Ling P., Kinchington P. R., Sadeghi-Zadeh M., Ruyechan W. T., Hay J. Transcription from varicella-zoster virus gene 67 (glycoprotein IV). J Virol. 1992 Jun;66(6):3690–3698. doi: 10.1128/jvi.66.6.3690-3698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetegui P., Campo-Vera H., Yamanishi K. Varicella-zoster virus (VZV)-specific polypeptides detected in cells treated with metabolic inhibitors. Microbiol Immunol. 1985;29(6):569–575. doi: 10.1111/j.1348-0421.1985.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Structural features of the herpes simplex virus alpha gene 4, 0, and 27 promoter-regulatory sequences which confer alpha regulation on chimeric thymidine kinase genes. J Virol. 1982 Dec;44(3):939–949. doi: 10.1128/jvi.44.3.939-949.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Campbell M. E., Haarr L., Frame M. C., Parris D. S., Murphy M., Hope R. G., Muller M. T., Preston C. M. The 65,000-Mr DNA-binding and virion trans-inducing proteins of herpes simplex virus type 1. J Virol. 1987 Aug;61(8):2428–2437. doi: 10.1128/jvi.61.8.2428-2437.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M. A., Hart R. P., Wong W. W., Nevins J. R. Sequences capable of restoring poly(A) site function define two distinct downstream elements. EMBO J. 1986 Nov;5(11):2907–2913. doi: 10.1002/j.1460-2075.1986.tb04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McKee T. A., Disney G. H., Everett R. D., Preston C. M. Control of expression of the varicella-zoster virus major immediate early gene. J Gen Virol. 1990 Apr;71(Pt 4):897–906. doi: 10.1099/0022-1317-71-4-897. [DOI] [PubMed] [Google Scholar]
- McKee T. A., Preston C. M. Identification of two protein binding sites within the varicella-zoster virus major immediate early gene promoter. Virus Res. 1991 Jun;20(1):59–69. doi: 10.1016/0168-1702(91)90061-y. [DOI] [PubMed] [Google Scholar]
- McKnight J. L., Kristie T. M., Roizman B. Binding of the virion protein mediating alpha gene induction in herpes simplex virus 1-infected cells to its cis site requires cellular proteins. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7061–7065. doi: 10.1073/pnas.84.20.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Smith H. A., Straus S. E., Cohen J. I. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992 Dec;66(12):7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Straus S. E., Cohen J. I. Varicella-zoster virus open reading frame 10 protein, the herpes simplex virus VP16 homolog, transactivates herpesvirus immediate-early gene promoters. J Virol. 1993 May;67(5):2739–2746. doi: 10.1128/jvi.67.5.2739-2746.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Ostrove J. M. Characterization of a potent varicella-zoster virus-encoded trans-repressor. J Virol. 1991 Oct;65(10):5289–5296. doi: 10.1128/jvi.65.10.5289-5296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R., Haigh A. Direct combinatorial interaction between a herpes simplex virus regulatory protein and a cellular octamer-binding factor mediates specific induction of virus immediate-early gene expression. EMBO J. 1988 Dec 20;7(13):4231–4238. doi: 10.1002/j.1460-2075.1988.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Comparison of upstream sequence requirements for positive and negative regulation of a herpes simplex virus immediate-early gene by three virus-encoded trans-acting factors. J Virol. 1987 Jan;61(1):190–199. doi: 10.1128/jvi.61.1.190-199.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrove J. M., Reinhold W., Fan C. M., Zorn S., Hay J., Straus S. E. Transcription mapping of the varicella-zoster virus genome. J Virol. 1985 Nov;56(2):600–606. doi: 10.1128/jvi.56.2.600-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera L. P., Mosca J. D., Ruyechan W. T., Hay J. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J Virol. 1992 Sep;66(9):5298–5304. doi: 10.1128/jvi.66.9.5298-5304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera L. P., Mosca J. D., Sadeghi-Zadeh M., Ruyechan W. T., Hay J. The varicella-zoster virus immediate early protein, IE62, can positively regulate its cognate promoter. Virology. 1992 Nov;191(1):346–354. doi: 10.1016/0042-6822(92)90197-w. [DOI] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Abnormal properties of an immediate early polypeptide in cells infected with the herpes simplex virus type 1 mutant tsK. J Virol. 1979 Nov;32(2):357–369. doi: 10.1128/jvi.32.2.357-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Reinhold W. C., Straus S. E., Ostrove J. M. Directionality and further mapping of varicella zoster virus transcripts. Virus Res. 1988 Feb;9(2-3):249–261. doi: 10.1016/0168-1702(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Clements J. B. Detailed structural analysis of two spliced HSV-1 immediate-early mRNAs. Nucleic Acids Res. 1982 Apr 10;10(7):2241–2256. doi: 10.1093/nar/10.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K., Hyman R. W. The immediate early proteins of varicella-zoster virus. Virology. 1987 Feb;156(2):423–426. doi: 10.1016/0042-6822(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Stamatatos L., Leventis R., Zuckermann M. J., Silvius J. R. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry. 1988 May 31;27(11):3917–3925. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- Stevenson D., Colman K. L., Davison A. J. Characterization of the varicella-zoster virus gene 61 protein. J Gen Virol. 1992 Mar;73(Pt 3):521–530. doi: 10.1099/0022-1317-73-3-521. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Aulakh H. S., Ruyechan W. T., Hay J., Casey T. A., Vande Woude G. F., Owens J., Smith H. A. Structure of varicella-zoster virus DNA. J Virol. 1981 Nov;40(2):516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triezenberg S. J., LaMarco K. L., McKnight S. L. Evidence of DNA: protein interactions that mediate HSV-1 immediate early gene activation by VP16. Genes Dev. 1988 Jun;2(6):730–742. doi: 10.1101/gad.2.6.730. [DOI] [PubMed] [Google Scholar]
- White R. J., Jackson S. P. The TATA-binding protein: a central role in transcription by RNA polymerases I, II and III. Trends Genet. 1992 Aug;8(8):284–288. doi: 10.1016/0168-9525(92)90255-3. [DOI] [PubMed] [Google Scholar]
- apRhys C. M., Ciufo D. M., O'Neill E. A., Kelly T. J., Hayward G. S. Overlapping octamer and TAATGARAT motifs in the VF65-response elements in herpes simplex virus immediate-early promoters represent independent binding sites for cellular nuclear factor III. J Virol. 1989 Jun;63(6):2798–2812. doi: 10.1128/jvi.63.6.2798-2812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]