Fig. 6.
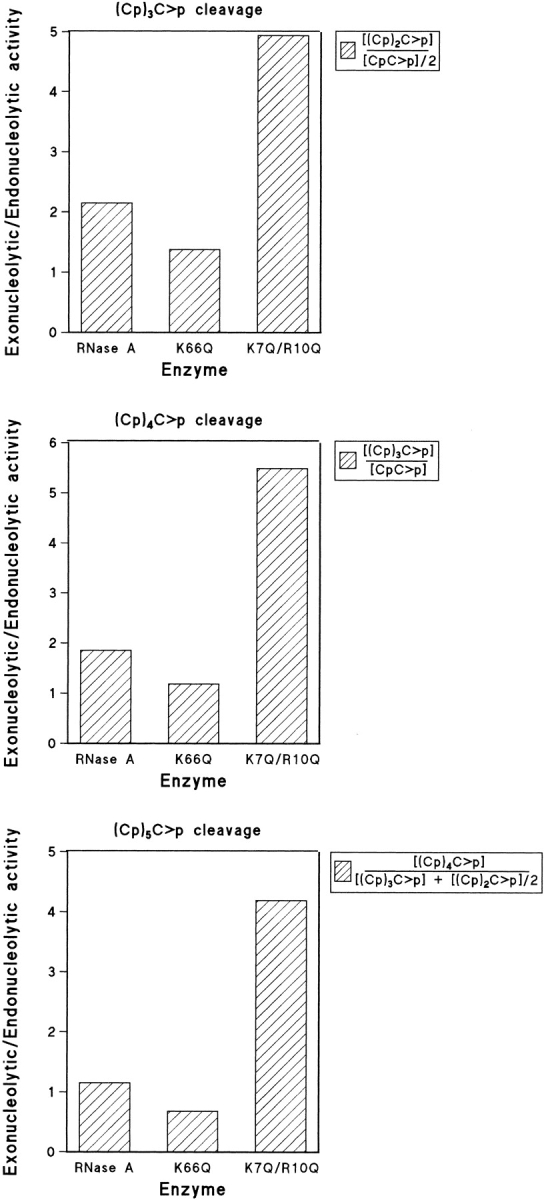
Exonucleolytic versus endonucleolytic activity of RNase A, K66Q-RNase A, and K7Q/R10Q-RNase A as determined from the ratios of the products at zero time. For the tetranucleotide substrate, the ratio of exonucleolytic to endonucleolytic cleavage was obtained as described in Figure 5 ▶. For the pentanucleotide substrate, the ratio was obtained by dividing the tetranucleotide formed by the dinucleotide formed, as each endonucleolytic cleavage gives rise to only one molecule of dinucleotide (the trinucleotide molecule could have been used as well). For the hexanucleotide substrate, endonucleolytic cleavage can result in either a tetranucleotide plus a dinucleotide or in two trinucleotides. Thus, the ratio was obtained by dividing the pentanucleotide formed (exonucleolytic cleavage) by the sum of tetranucleotide plus half of the trinucleotide formed.
