Abstract
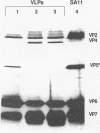
Rotaviruses are triple-layered particles that contain four major capsid proteins, VP2, VP4, VP6, and VP7, and two minor proteins, VP1 and VP3. We have cloned each of the rotavirus genes coding for a major capsid protein into the baculovirus expression system and expressed each protein in insect cells. Coexpression of different combinations of the rotavirus major structural proteins resulted in the formation of stable virus-like particles (VLPs). The coexpression of VP2 and VP6 alone or with VP4 resulted in the production of VP2/6 or VP2/4/6 VLPs, which were similar to double-layered rotavirus particles. Coexpression of VP2, VP6, and VP7, with or without VP4, produced triple-layered VP2/6/7 or VP2/4/6/7 VLPs, which were similar to native infectious rotavirus particles. The VLPs maintained the structural and functional characteristics of native particles, as determined by electron microscopic examination of the particles, the presence of nonneutralizing and neutralizing epitopes on VP4 and VP7, and hemagglutination activity of the VP2/4/6/7 VLPs. The production of VP2/4/6 particles indicated that VP4 interacts with VP6. Cell binding assays performed with each of the VLPs indicated that VP4 is the viral attachment protein. Chimeric particles containing VP7 from two different G serotypes also were obtained. The ability to express individual proteins or to coexpress different subsets of proteins provides a system with which to examine the interactions of the rotavirus structural proteins, the role of individual proteins in virus morphogenesis, and the feasibility of a subunit vaccine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar R. L., Metcalf T. G., Neill F. H., Estes M. K. Detection of enteric viruses in oysters by using the polymerase chain reaction. Appl Environ Microbiol. 1993 Feb;59(2):631–635. doi: 10.1128/aem.59.2.631-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au K. S., Chan W. K., Burns J. W., Estes M. K. Receptor activity of rotavirus nonstructural glycoprotein NS28. J Virol. 1989 Nov;63(11):4553–4562. doi: 10.1128/jvi.63.11.4553-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. M., Mackow E. R., Greenberg H. B. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991 Aug;183(2):602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- Bass D. M., Mackow E. R., Greenberg H. B. NS35 and not vp7 is the soluble rotavirus protein which binds to target cells. J Virol. 1990 Jan;64(1):322–330. doi: 10.1128/jvi.64.1.322-330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bican P., Cohen J., Charpilienne A., Scherrer R. Purification and characterization of bovine rotavirus cores. J Virol. 1982 Sep;43(3):1113–1117. doi: 10.1128/jvi.43.3.1113-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. W., Greenberg H. B., Shaw R. D., Estes M. K. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J Virol. 1988 Jun;62(6):2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadd T. L., Patterson J. L. Synthesis of viruslike particles by expression of the putative capsid protein of Leishmania RNA virus in a recombinant baculovirus expression system. J Virol. 1994 Jan;68(1):358–365. doi: 10.1128/jvi.68.1.358-365.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Y., Estes M. K., Ramig R. F. Specific interactions between rotavirus outer capsid proteins VP4 and VP7 determine expression of a cross-reactive, neutralizing VP4-specific epitope. J Virol. 1992 Jan;66(1):432–439. doi: 10.1128/jvi.66.1.432-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Burns J. W., Estes M. K., Ramig R. F. Phenotypes of rotavirus reassortants depend upon the recipient genetic background. Proc Natl Acad Sci U S A. 1989 May;86(10):3743–3747. doi: 10.1073/pnas.86.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., White J. R., Cotton R. G. Non-neutralizing monoclonal antibodies to a trypsin-sensitive site on the major glycoprotein of rotavirus which discriminate between virus serotypes. Arch Virol. 1987;93(3-4):199–211. doi: 10.1007/BF01310974. [DOI] [PubMed] [Google Scholar]
- Dormitzer P. R., Greenberg H. B. Calcium chelation induces a conformational change in recombinant herpes simplex virus-1-expressed rotavirus VP7. Virology. 1992 Aug;189(2):828–832. doi: 10.1016/0042-6822(92)90616-w. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Identification of rotaviruses of different origins by the plaque-reduction test. Am J Vet Res. 1980 Jan;41(1):151–152. [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Ramig R. F., Ericson B. L. Heterogeneity in the structural glycoprotein (VP7) of simian rotavirus SA11. Virology. 1982 Oct 15;122(1):8–14. doi: 10.1016/0042-6822(82)90372-5. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Mason B. B., Crawford S., Cohen J. Cloning and nucleotide sequence of the simian rotavirus gene 6 that codes for the major inner capsid protein. Nucleic Acids Res. 1984 Feb 24;12(4):1875–1887. doi: 10.1093/nar/12.4.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore L., Greenberg H. B., Mackow E. R. The VP8 fragment of VP4 is the rhesus rotavirus hemagglutinin. Virology. 1991 Apr;181(2):553–563. doi: 10.1016/0042-6822(91)90888-i. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T. Role of VP3 in human rotavirus internalization after target cell attachment via VP7. J Virol. 1988 Jul;62(7):2209–2218. doi: 10.1128/jvi.62.7.2209-2218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. S., Boulanger P. Assembly-defective point mutants of the human immunodeficiency virus type 1 Gag precursor phenotypically expressed in recombinant baculovirus-infected cells. J Virol. 1993 May;67(5):2787–2798. doi: 10.1128/jvi.67.5.2787-2798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang M., Graham D. Y., Estes M. K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992 Nov;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Midthun K., Hoshino Y., Green K. Y., Gorziglia M., Nishikawa K., Chanock R. M., Potash L., Perez-Schael I. Strategies for the development of a rotavirus vaccine against infantile diarrhea with an update on clinical trials of rotavirus vaccines. Adv Exp Med Biol. 1989;257:67–89. doi: 10.1007/978-1-4684-5712-4_9. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R., Taub J., Greenstone H., Roden R., Dürst M., Gissmann L., Lowy D. R., Schiller J. T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993 Dec;67(12):6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé M., Charpilienne A., Crawford S. E., Estes M. K., Cohen J. Expression of rotavirus VP2 produces empty corelike particles. J Virol. 1991 Jun;65(6):2946–2952. doi: 10.1128/jvi.65.6.2946-2952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blois H., Roy P. A single point mutation in the VP7 major core protein of bluetongue virus prevents the formation of core-like particles. J Virol. 1993 Jan;67(1):353–359. doi: 10.1128/jvi.67.1.353-359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizano M., López S., Arias C. F. The amino-terminal half of rotavirus SA114fM VP4 protein contains a hemagglutination domain and primes for neutralizing antibodies to the virus. J Virol. 1991 Mar;65(3):1383–1391. doi: 10.1128/jvi.65.3.1383-1391.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon P. T., Roy P. Assembly of five bluetongue virus proteins expressed by recombinant baculoviruses: inclusion of the largest protein VP1 in the core and virus-like proteins. Virology. 1991 Feb;180(2):798–802. doi: 10.1016/0042-6822(91)90094-r. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Barnett J. W., Chan H., Greenberg H. B. The rhesus rotavirus outer capsid protein VP4 functions as a hemagglutinin and is antigenically conserved when expressed by a baculovirus recombinant. J Virol. 1989 Apr;63(4):1661–1668. doi: 10.1128/jvi.63.4.1661-1668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. Biochemical mapping of the simian rotavirus SA11 genome. J Virol. 1983 May;46(2):413–423. doi: 10.1128/jvi.46.2.413-423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson D. O., Estes M. K., Burns J. W., Greenberg H. B., Taniguchi K., Urasawa S. Serotype variation of human group A rotaviruses in two regions of the USA. J Infect Dis. 1990 Sep;162(3):605–614. doi: 10.1093/infdis/162.3.605. [DOI] [PubMed] [Google Scholar]
- Méndez E., Arias C. F., López S. Binding to sialic acids is not an essential step for the entry of animal rotaviruses to epithelial cells in culture. J Virol. 1993 Sep;67(9):5253–5259. doi: 10.1128/jvi.67.9.5253-5259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Ready K. F., Sabara M. In vitro assembly of bovine rotavirus nucleocapsid protein. Virology. 1987 Mar;157(1):189–198. doi: 10.1016/0042-6822(87)90328-x. [DOI] [PubMed] [Google Scholar]
- Redmond M. J., Ijaz M. K., Parker M. D., Sabara M. I., Dent D., Gibbons E., Babiuk L. A. Assembly of recombinant rotavirus proteins into virus-like particles and assessment of vaccine potential. Vaccine. 1993;11(2):273–281. doi: 10.1016/0264-410x(93)90029-w. [DOI] [PubMed] [Google Scholar]
- Ruggeri F. M., Greenberg H. B. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991 May;65(5):2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J. E., Hudson G. R., Babiuk L. A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985 Jan;53(1):58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. L., Rothnagel R., Chen D., Ramig R. F., Chiu W., Prasad B. V. Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell. 1993 Aug 27;74(4):693–701. doi: 10.1016/0092-8674(93)90516-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Greenberg H. B. Phenotypic mixing during coinfection of cells with two strains of human rotavirus. J Virol. 1988 Nov;62(11):4358–4361. doi: 10.1128/jvi.62.11.4358-4361.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C. Q., Labbé M., Cohen J., Prasad B. V., Chen D., Ramig R. F., Estes M. K. Characterization of rotavirus VP2 particles. Virology. 1994 May 15;201(1):55–65. doi: 10.1006/viro.1994.1265. [DOI] [PubMed] [Google Scholar]
- Zhou Y. J., Burns J. W., Morita Y., Tanaka T., Estes M. K. Localization of rotavirus VP4 neutralization epitopes involved in antibody-induced conformational changes of virus structure. J Virol. 1994 Jun;68(6):3955–3964. doi: 10.1128/jvi.68.6.3955-3964.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]