Abstract
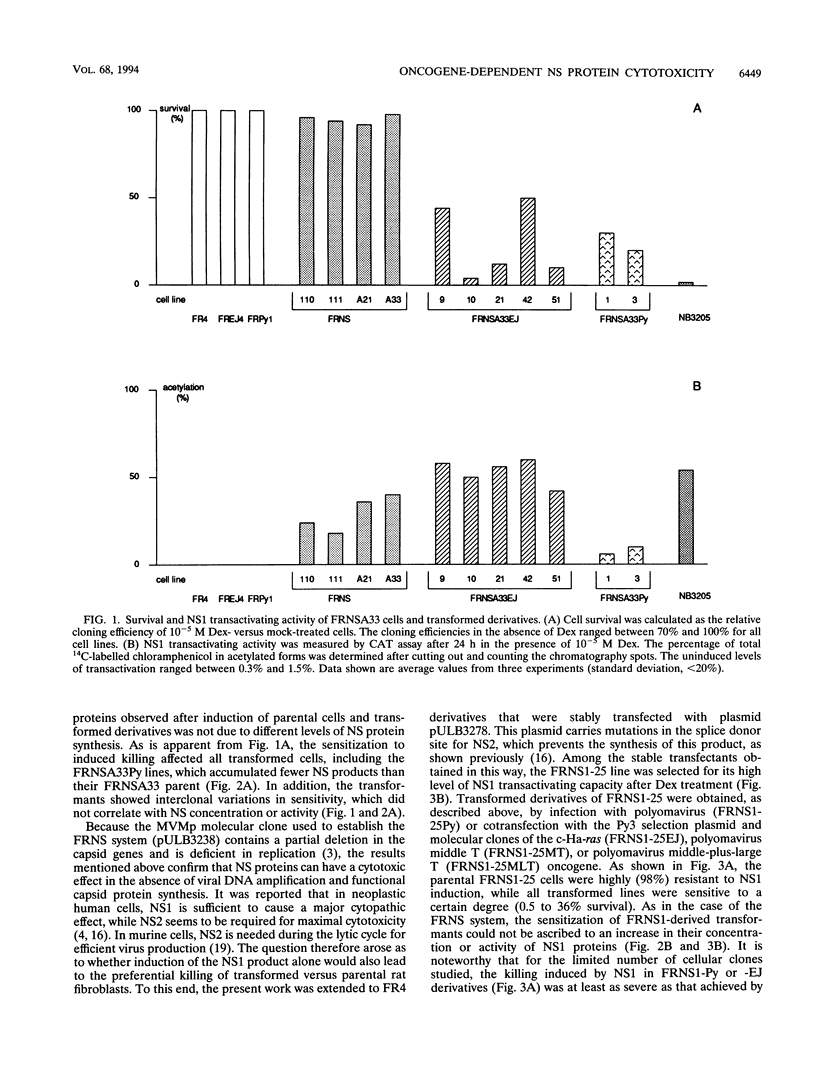
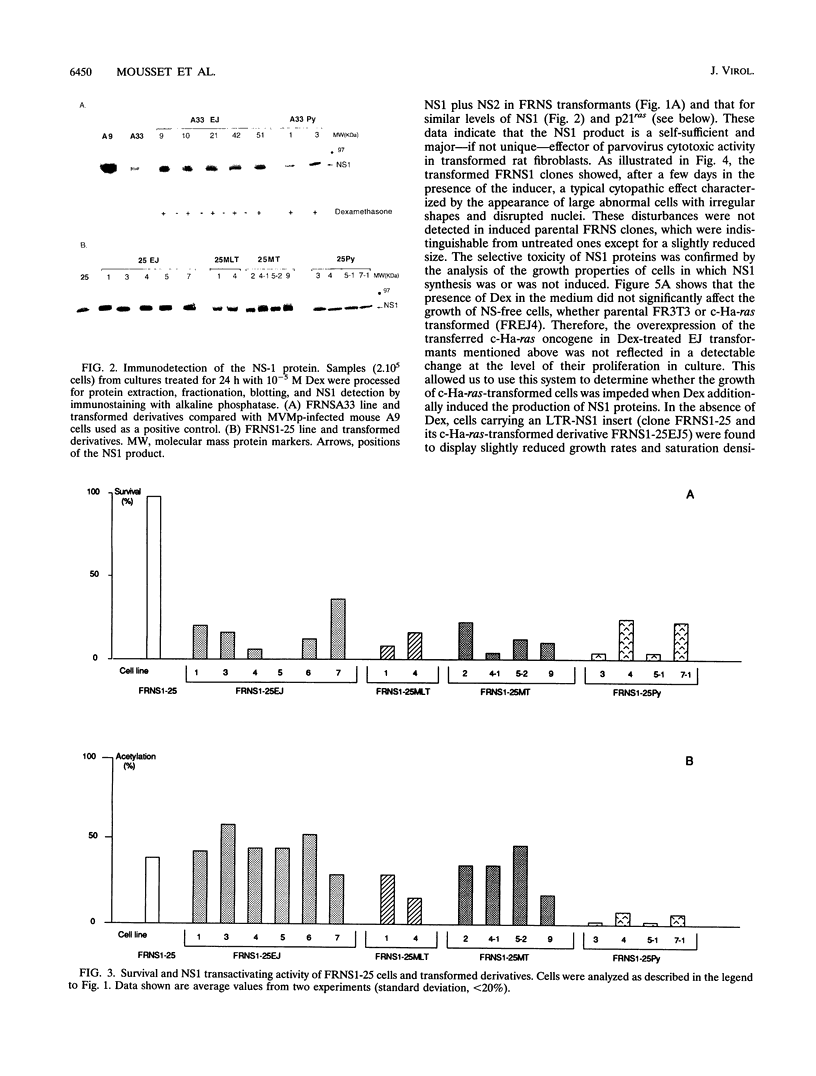
The nonstructural (NS) proteins of the autonomous parvovirus minute virus of mice are involved in viral DNA replication and in the regulation of homologous and heterologous promoters. Moreover, NS products have proved to be cytotoxic, especially for transformed cells. We show here that intracellular accumulation of NS products is not sufficient to kill rat fibroblasts from the established cell line FR3T3, which is phenotypically normal in several respects. FRNS cell lines were obtained by stable transfection of FR3T3 cells by a vector carrying the NS genes under the control of the hormone-inducible long terminal repeat promoter of the mouse mammary tumor virus. In the presence of dexamethasone, the NS proteins were synthesized without associated cell death. Transformation of FRNS cells with the c-Ha-ras oncogene or polyomavirus oncogenes had little effect on their capacity for NS induction, as measured at both concentration and transactivating activity levels, yet the transformants were now dying within a few days in the presence of the inducer. The same results were obtained with cells stably transfected by a vector expressing the NS1 product alone, suggesting that in this system there is no cooperation between NS1 and NS2 for maximal cytopathic effect. Cell mortality after NS protein induction was quantitatively related to the yield of oncogene expression, while NS-1 was not limiting in this respect. Our results show that the NS1 protein is not lethal unless cellular factors that may depend on oncogene expression trigger its cytotoxicity.
Full text
PDF


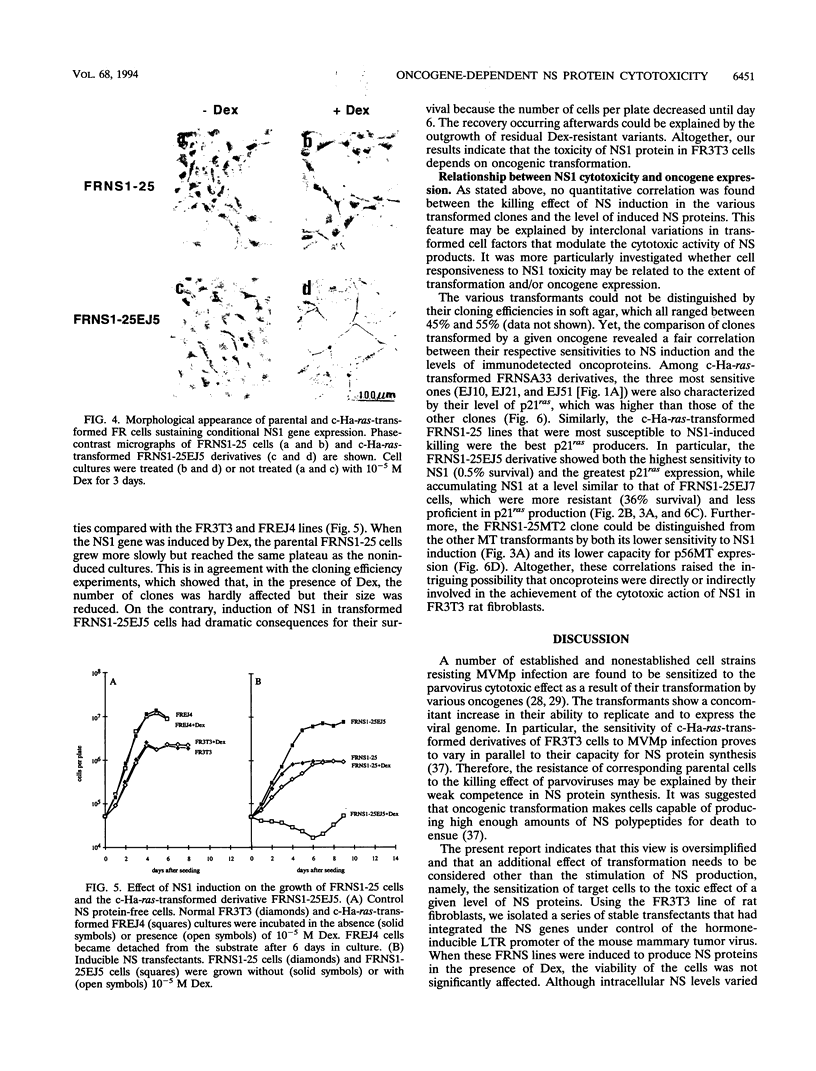


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselin C., Vass-Marengo J., Bastin M. Mutation in the polyomavirus genome that activates the properties of large T associated with neoplastic transformation. J Virol. 1986 Jan;57(1):165–172. doi: 10.1128/jvi.57.1.165-172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy R., Astell C. R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5'-terminal palindrome of minute virus of mice. Gene. 1985;35(1-2):179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- Brandenburger A., Legendre D., Avalosse B., Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990 Feb;174(2):576–584. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Perros M., Brandenburger A., Spegelaere P., Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990 Sep;9(9):2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis J. J., Spruyt N., Spegelaere P., Guetta E., Darawshi T., Cotmore S. F., Tal J., Rommelaere J. Sensitization of transformed rat fibroblasts to killing by parvovirus minute virus of mice correlates with an increase in viral gene expression. J Virol. 1988 Sep;62(9):3438–3444. doi: 10.1128/jvi.62.9.3438-3444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Organization of nonstructural genes of the autonomous parvovirus minute virus of mice. J Virol. 1986 Jun;58(3):724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Antonietti J. P., Beard P. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J Virol. 1990 Jan;64(1):387–396. doi: 10.1128/jvi.64.1.387-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas C., Schaffhausen B., Bockus B., Ratiarson A., Bastin M. Mutations in polyomavirus middle T antigen affecting tumorigenesis. Virology. 1989 May;170(1):193–200. doi: 10.1016/0042-6822(89)90366-8. [DOI] [PubMed] [Google Scholar]
- Gu M. L., Chen F. X., Rhode S. L. Parvovirus H-1 P38 promoter requires the trans-activation region (tar), an SP1 site, and a TATA box for full activity. Virology. 1992 Mar;187(1):10–17. doi: 10.1016/0042-6822(92)90290-6. [DOI] [PubMed] [Google Scholar]
- Guetta E., Mincberg M., Mousset S., Bertinchamps C., Rommelaere J., Tal J. Selective killing of transformed rat cells by minute virus of mice does not require infectious virus production. J Virol. 1990 Jan;64(1):458–462. doi: 10.1128/jvi.64.1.458-462.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre D., Rommelaere J. Terminal regions of the NS-1 protein of the parvovirus minute virus of mice are involved in cytotoxicity and promoter trans inhibition. J Virol. 1992 Oct;66(10):5705–5713. doi: 10.1128/jvi.66.10.5705-5713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand C., Mousset S., Salomé N., Rommelaere J. Cooperation of oncogenes in cell transformation and sensitization to killing by the parvovirus minute virus of mice. J Gen Virol. 1992 Aug;73(Pt 8):2003–2009. doi: 10.1099/0022-1317-73-8-2003. [DOI] [PubMed] [Google Scholar]
- Legrand C., Rommelaere J., Caillet-Fauquet P. MVM(p) NS-2 protein expression is required with NS-1 for maximal cytotoxicity in human transformed cells. Virology. 1993 Jul;195(1):149–155. doi: 10.1006/viro.1993.1355. [DOI] [PubMed] [Google Scholar]
- Mousset S., Cornelis J., Spruyt N., Rommelaere J. Transformation of established murine fibroblasts with an activated cellular Harvey-ras oncogene or the polyoma virus middle T gene increases cell permissiveness to parvovirus minute-virus-of-mice. Biochimie. 1986 Jul-Aug;68(7-8):951–955. doi: 10.1016/s0300-9084(86)81058-6. [DOI] [PubMed] [Google Scholar]
- Mousset S., Rommelaere J. Minute virus of mice inhibits cell transformation by simian virus 40. Nature. 1982 Dec 9;300(5892):537–539. doi: 10.1038/300537a0. [DOI] [PubMed] [Google Scholar]
- Naeger L. K., Cater J., Pintel D. J. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990 Dec;64(12):6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeger L. K., Salomé N., Pintel D. J. NS2 is required for efficient translation of viral mRNA in minute virus of mice-infected murine cells. J Virol. 1993 Feb;67(2):1034–1043. doi: 10.1128/jvi.67.2.1034-1043.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Construction of a genetic switch for inducible trans-activation of gene expression in eucaryotic cells. J Virol. 1987 May;61(5):1448–1456. doi: 10.1128/jvi.61.5.1448-1456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Richard S. M. Characterization of the trans-activation-responsive element of the parvovirus H-1 P38 promoter. J Virol. 1987 Sep;61(9):2807–2815. doi: 10.1128/jvi.61.9.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Cornelis J. J. Antineoplastic activity of parvoviruses. J Virol Methods. 1991 Aug;33(3):233–251. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- Salome N., van Hille B., Geuskens M., Rommelaere J. Partial reversion of conditional transformation correlates with a decrease in the sensitivity of rat cells to killing by the parvovirus minute virus of mice but not in their capacity for virus production: effect of a temperature-sensitive v-src oncogene. J Virol. 1989 Nov;63(11):4797–4807. doi: 10.1128/jvi.63.11.4797-4807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé N., van Hille B., Duponchel N., Meneguzzi G., Cuzin F., Rommelaere J., Cornelis J. J. Sensitization of transformed rat cells to parvovirus MVMp is restricted to specific oncogenes. Oncogene. 1990 Jan;5(1):123–130. [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Strawhecker J. M., Betz N. A., Neades R. Y., Houser W., Pelling J. C. Binding of the 97 kDa glucocorticoid receptor to the 5' upstream flanking region of the mouse c-Ha-ras oncogene. Oncogene. 1989 Nov;4(11):1317–1322. [PubMed] [Google Scholar]
- Van Hille B., Duponchel N., Salomé N., Spruyt N., Cotmore S. F., Tattersall P., Cornelis J. J., Rommelaere J. Limitations to the expression of parvoviral nonstructural proteins may determine the extent of sensitization of EJ-ras-transformed rat cells to minute virus of mice. Virology. 1989 Jul;171(1):89–97. doi: 10.1016/0042-6822(89)90514-x. [DOI] [PubMed] [Google Scholar]