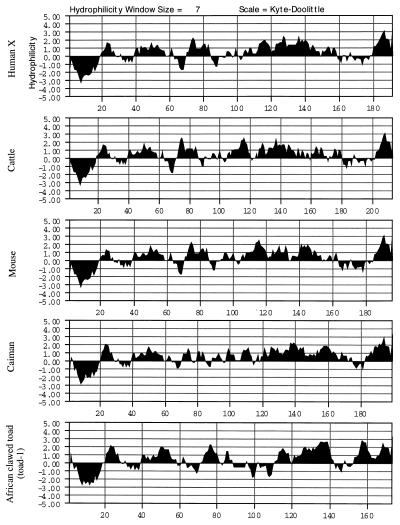
Figure 3.
Hydrophilicity plot of amelogenins from selected species prepared by using the method of Kyte and Doolittle (25). The plots share the following characteristics: the hydrophobic leader peptide of about 20 amino acid residues is followed by a short hydrophilic segment (10 residues), another short hydrophobic segment (10 residues), and a larger hydrophilic segment (about 20 residues). The conserved α-helical segment lies on the border of the hydrophobic and hydrophilic domains (approximately corresponding to residues 35–45). This shared hydrophobicity signature is followed by a more variable internal segment (residues 60–110) of irregularly alternating hydrophilicity and hydrophobicity. The repeat region (residues 110–190) and the C-terminal region are hydrophilic. Although the primary sequence of amelogenin varies greatly between species, the hydropathy pattern is well conserved.