Abstract
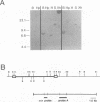
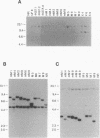
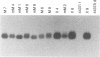
In earlier studies, we have shown that superinfection of an interleukin-2 (IL-2)-dependent, Moloney murine leukemia virus (MoMuLV)-induced rat T-cell lymphoma line (4437A) with mink cell focus-forming (also called polytropic) murine retroviruses induces rapid progression to IL-2-independent growth. In this report, we present evidence that the vast majority (> 90%) of the IL-2-independent lines established from polytropic or xenotropic virus-infected 4437A cells carry provirus insertions in the 3' untranslated region of the IL-9 receptor gene (Gfi-2 [for growth factor independence-2]/IL-9R). Prior to superinfection, the cells express neither IL-9 nor IL-9R. Following superinfection and provirus insertion in the Gfi-2/IL-9R locus, the cells express high levels of mRNA transcripts with a truncated 3' untranslated region which are predicted to encode the normal IL-9R protein product. The same IL-2-independent cells also express IL-9 which is induced by an insertional mutagenesis-independent mechanism. The establishment of an IL-9-dependent autocrine loop was sufficient to render the cells IL-2 independent, as suggested by the finding that 4437A cells, expressing a stably transfected Gfi-2/IL-9R construct, do not require IL-2 when maintained in IL-9-containing media. Additional experiments designed on the basis of these results showed that IL-9 gene expression is induced rapidly following the infection of 4437A cells by polytropic or xenotropic viruses and occurs in the absence of selection for IL-2-independent growth. Taken together, these data suggest that infection of 4437A cells by mink cell focus-forming or xenotropic viruses induces the expression of IL-9, which in turn rapidly selects the cells expressing the IL-9 receptor through an insertional mutagenesis-dependent mechanism. Given that both the polytropic and xenotropic viruses can induce the IL-9-dependent autocrine loop, the reduced ability of the xenotropic viruses to rapidly induce IL-2 independence in culture and tumors in animals is likely to be the result of their lower growth rates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Barker C. S., Bear S. E., Keler T., Copeland N. G., Gilbert D. J., Jenkins N. A., Yeung R. S., Tsichlis P. N. Activation of the prolactin receptor gene by promoter insertion in a Moloney murine leukemia virus-induced rat thymoma. J Virol. 1992 Nov;66(11):6763–6768. doi: 10.1128/jvi.66.11.6763-6768.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A., Lazo P. A., Bear S. E., Tsichlis P. N. The rat leukocyte antigen MRC OX-44 is a member of a new family of cell surface proteins which appear to be involved in growth regulation. Mol Cell Biol. 1991 May;11(5):2864–2872. doi: 10.1128/mcb.11.5.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman B. K., Davis B. R., Fan H. Preleukemic hematopoietic hyperplasia induced by Moloney murine leukemia virus is an indirect consequence of viral infection. J Virol. 1990 Sep;64(9):4582–4584. doi: 10.1128/jvi.64.9.4582-4584.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988 Jul-Aug;6(7):632–638. [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Malim M. H. The HIV-1 Rev protein: prototype of a novel class of eukaryotic post-transcriptional regulators. Trends Biochem Sci. 1991 Sep;16(9):346–350. doi: 10.1016/0968-0004(91)90141-h. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Mechanism of action of regulatory proteins encoded by complex retroviruses. Microbiol Rev. 1992 Sep;56(3):375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. R., Brightman B. K., Chandy K. G., Fan H. Characterization of a preleukemic state induced by Moloney murine leukemia virus: evidence for two infection events during leukemogenesis. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4875–4879. doi: 10.1073/pnas.84.14.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. R., Chandy K. G., Brightman B. K., Gupta S., Fan H. Effects of nonleukemogenic and wild-type Moloney murine leukemia virus on lymphoid cells in vivo: identification of a preleukemic shift in thymocyte subpopulations. J Virol. 1986 Nov;60(2):423–430. doi: 10.1128/jvi.60.2.423-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990 Feb;124(2):221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. Genetic identification of endogenous polytropic proviruses by using recombinant inbred mice. J Virol. 1989 Sep;63(9):3810–3821. doi: 10.1128/jvi.63.9.3810-3821.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks C. B., Bear S. E., Grimes H. L., Tsichlis P. N. Progression of interleukin-2 (IL-2)-dependent rat T cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. Mol Cell Biol. 1993 Mar;13(3):1759–1768. doi: 10.1128/mcb.13.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Andresson T., Sparkowski J. J., Schlegel R. The BPV-1 E5 protein, the 16 kDa membrane pore-forming protein and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions. EMBO J. 1992 Dec;11(13):4851–4859. doi: 10.1002/j.1460-2075.1992.tb05591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon M. L., Colizzi V., Dalgleish A., Montagnier L. New concepts in AIDS pathogenesis. AIDS Res Hum Retroviruses. 1993 Mar;9(3):287–289. doi: 10.1089/aid.1993.9.287. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiai H., Morrissey P., Khiroya R., Schwartz R. S. Selective expression of xenotropic virus in congenic HRS/J (hairless) mice. Nature. 1977 Nov 17;270(5634):247–249. doi: 10.1038/270247a0. [DOI] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Thomas C. Y., Chattopadhyay S. K., Koehne C., O'Donnell P. V. Influence of enhancer sequences on thymotropism and leukemogenicity of mink cell focus-forming viruses. J Virol. 1989 Mar;63(3):1284–1292. doi: 10.1128/jvi.63.3.1284-1292.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Pallas D. C., Morgan W., Schaffhausen B., Roberts T. M. Mechanisms of transformation by polyoma virus middle T antigen. Biochim Biophys Acta. 1989 Feb;948(3):345–364. doi: 10.1016/0304-419x(89)90006-1. [DOI] [PubMed] [Google Scholar]
- Lazo P. A., Klein-Szanto A. J., Tsichlis P. N. T-cell lymphoma lines derived from rat thymomas induced by Moloney murine leukemia virus: phenotypic diversity and its implications. J Virol. 1990 Aug;64(8):3948–3959. doi: 10.1128/jvi.64.8.3948-3959.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- Li J. P., Baltimore D. Mechanism of leukemogenesis induced by mink cell focus-forming murine leukemia viruses. J Virol. 1991 May;65(5):2408–2414. doi: 10.1128/jvi.65.5.2408-2414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriotis C., Makris A., Bear S. E., Tsichlis P. N. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2251–2255. doi: 10.1073/pnas.90.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., DiMaio D. Stable association between the bovine papillomavirus E5 transforming protein and activated platelet-derived growth factor receptor in transformed mouse cells. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6736–6740. doi: 10.1073/pnas.89.15.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Nilson L. A., DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991 Apr;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Pavlakis G. N. Tat and Rev: positive regulators of HIV gene expression. AIDS. 1990 Jun;4(6):499–509. [PubMed] [Google Scholar]
- Tsichlis P. N., Bear S. E. Infection by mink cell focus-forming viruses confers interleukin 2 (IL-2) independence to an IL-2-dependent rat T-cell lymphoma line. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4611–4615. doi: 10.1073/pnas.88.11.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Lazo P. A. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr Top Microbiol Immunol. 1991;171:95–171. doi: 10.1007/978-3-642-76524-7_5. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C., Druez C., Renauld J. C., Hérin M., Noël H., Van Snick J. Autonomous growth and tumorigenicity induced by P40/interleukin 9 cDNA transfection of a mouse P40-dependent T cell line. J Exp Med. 1991 Feb 1;173(2):519–522. doi: 10.1084/jem.173.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. L., McGrath M. S. Retrovirus lymphomagenesis: relationship of normal immune receptors to malignant cell proliferation. Curr Top Microbiol Immunol. 1982;98:103–112. doi: 10.1007/978-3-642-68369-5_8. [DOI] [PubMed] [Google Scholar]