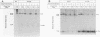Abstract
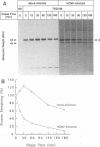
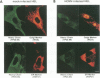
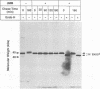
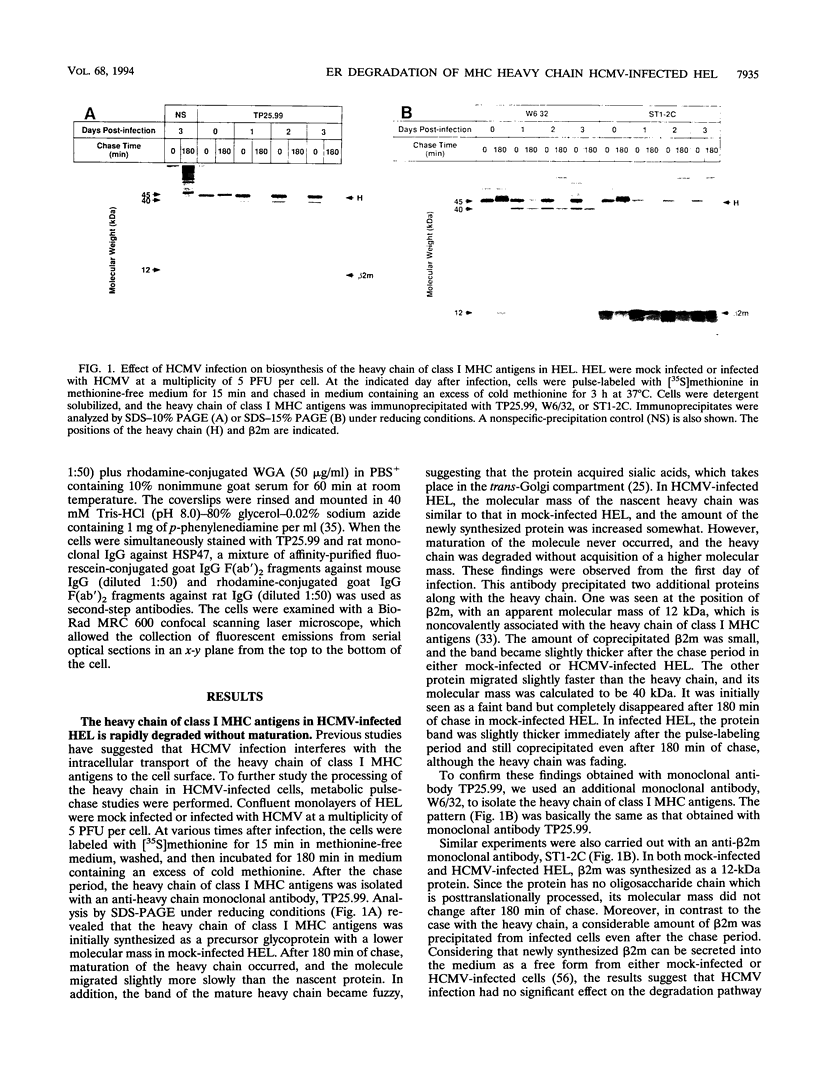
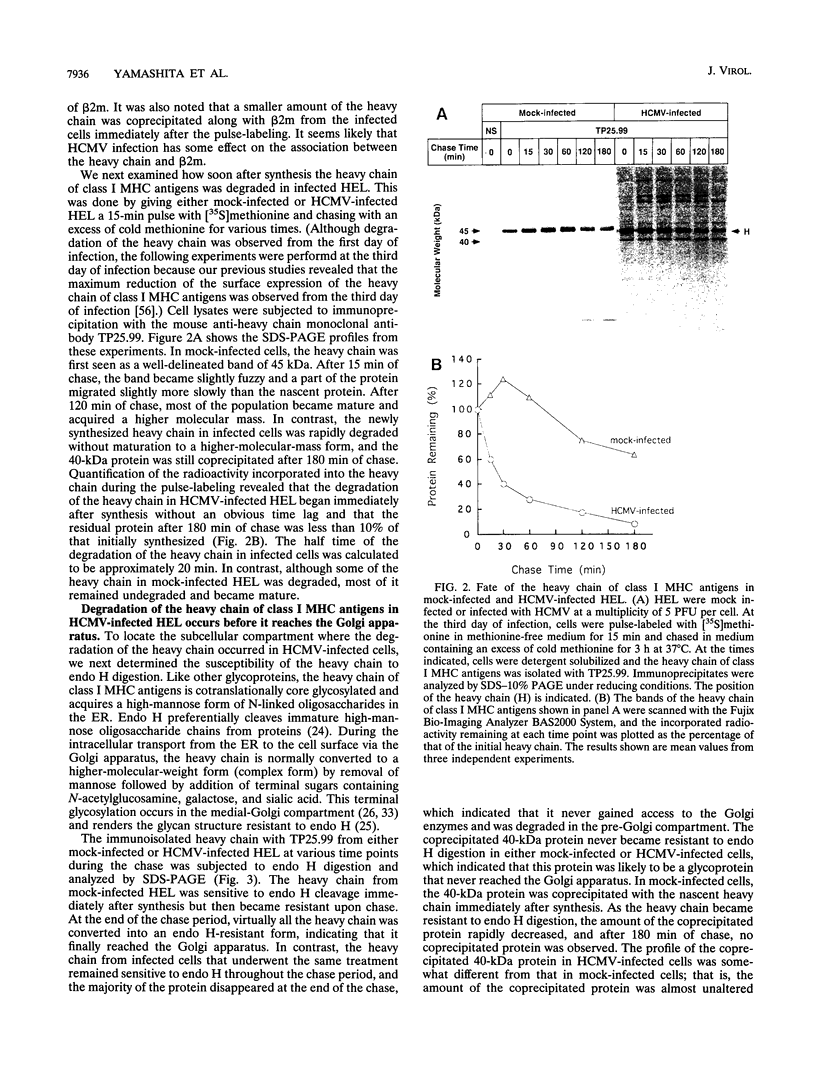
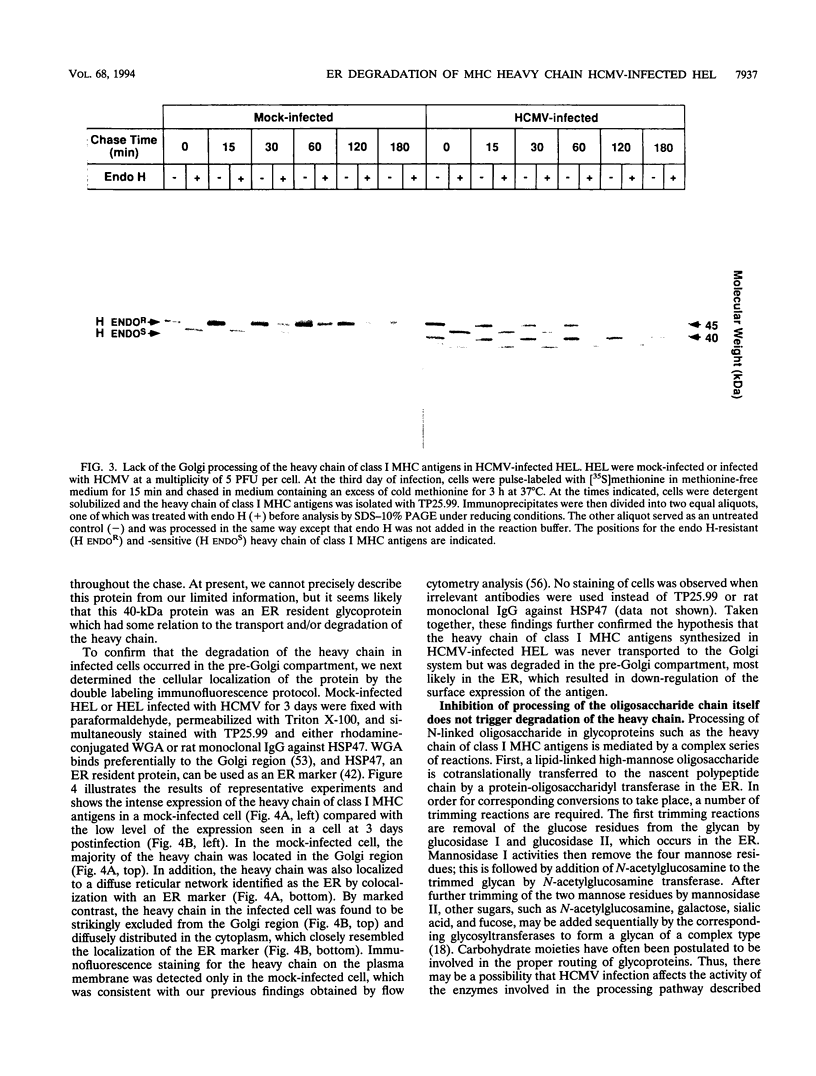
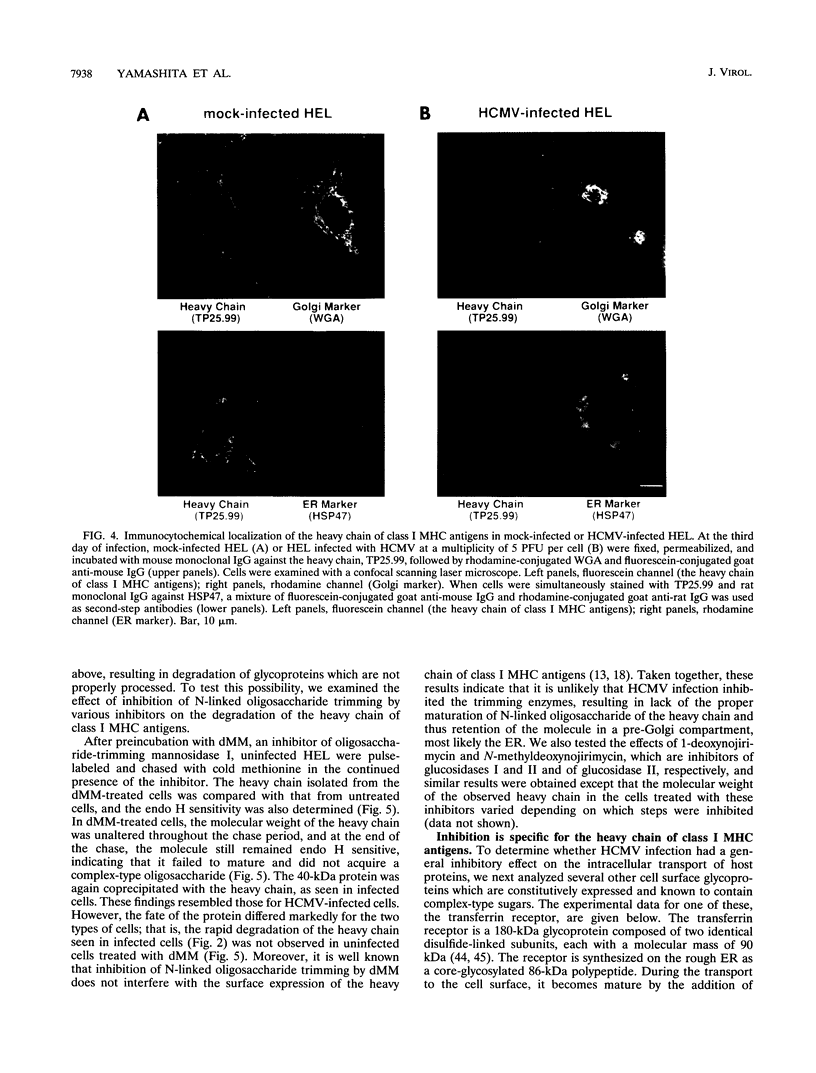
Human cytomegalovirus (HCMV) infection results in a marked reduction in the surface expression of class I major histocompatibility complex antigens on the host cells, which is thought to be one of the means for HCMV to evade the host immune system. To clarify the precise mechanism(s) of this phenomenon, we investigated the fate of the heavy chain of class I major histocompatibility complex antigens in HCMV-infected human embryonic lung fibroblasts (HEL) by pulse-chase analysis and immunocytochemical techniques. In HCMV-infected HEL, the heavy chain was synthesized at an increasing rate. However, instead of being transported to the cell surface through the Golgi apparatus, it was retained in the endoplasmic reticulum (ER) without acquisition of a complex-type N-linked oligosaccharide. In addition, it was rapidly degraded, with a half time of 20 min, and the amount of the heavy chain remaining at the end of 3 h of chase was 10% (or less) of that initially synthesized. ER degradation of host glycoproteins in HCMV-infected HEL was selective for the heavy chain, since the posttranslational processing of the transferrin receptor in these cells was not affected. The heavy chain degradation in infected cells was resistant to inhibitors of a lysosomal proteolytic pathway and to metabolic poisons. These observations suggest the presence of an energy-dependent nonlysosomal proteolytic pathway in the ER.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Barnes P. D., Grundy J. E. Down-regulation of the class I HLA heterodimer and beta 2-microglobulin on the surface of cells infected with cytomegalovirus. J Gen Virol. 1992 Sep;73(Pt 9):2395–2403. doi: 10.1099/0022-1317-73-9-2395. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Beck S., Barrell B. G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988 Jan 21;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Lippincott-Schwartz J., Chen C., Antusch D., Samelson L. E., Klausner R. D. Association and dissociation of the murine T cell receptor associated protein (TRAP). Early events in the biosynthesis of a multisubunit receptor. J Biol Chem. 1988 Jun 25;263(18):8965–8971. [PubMed] [Google Scholar]
- Bonifacino J. S., Suzuki C. K., Lippincott-Schwartz J., Weissman A. M., Klausner R. D. Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J Cell Biol. 1989 Jul;109(1):73–83. doi: 10.1083/jcb.109.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Vugler L. G. Processing of the gp55-116 envelope glycoprotein complex (gB) of human cytomegalovirus. J Virol. 1989 Jan;63(1):403–410. doi: 10.1128/jvi.63.1.403-410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne H., Churcher M., Minson T. Construction and characterization of a human cytomegalovirus mutant with the UL18 (class I homolog) gene deleted. J Virol. 1992 Nov;66(11):6784–6787. doi: 10.1128/jvi.66.11.6784-6787.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne H., Smith G., Beck S., Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature. 1990 Oct 25;347(6295):770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984 Mar;3(3):551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Bonifacino J. S., Yuan L. C., Klausner R. D. Selective degradation of T cell antigen receptor chains retained in a pre-Golgi compartment. J Cell Biol. 1988 Dec;107(6 Pt 1):2149–2161. doi: 10.1083/jcb.107.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Gonen H. The ubiquitin-mediated proteolytic pathway: enzymology and mechanisms of recognition of the proteolytic substrates. Semin Cell Biol. 1990 Dec;1(6):415–422. [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- D'Urso C. M., Wang Z. G., Cao Y., Tatake R., Zeff R. A., Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J Clin Invest. 1991 Jan;87(1):284–292. doi: 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Ploegh H. Inhibitors of oligosaccharide processing. Biochim Biophys Acta. 1985 Jun 24;825(2):95–110. doi: 10.1016/0167-4781(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Green S. A., Zimmer K. P., Griffiths G., Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987 Sep;105(3):1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., Ayles H. M., McKeating J. A., Butcher R. G., Griffiths P. D., Poulter L. W. Enhancement of class I HLA antigen expression by cytomegalovirus: role in amplification of virus infection. J Med Virol. 1988 Aug;25(4):483–495. doi: 10.1002/jmv.1890250412. [DOI] [PubMed] [Google Scholar]
- Hosenpud J. D., Chou S. W., Wagner C. R. Cytomegalovirus-induced regulation of major histocompatibility complex class I antigen expression in human aortic smooth muscle cells. Transplantation. 1991 Nov;52(5):896–903. doi: 10.1097/00007890-199111000-00027. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Bonifacino J. S., Yuan L. C., Klausner R. D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988 Jul 15;54(2):209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- Maudsley D. J., Pound J. D. Modulation of MHC antigen expression by viruses and oncogenes. Immunol Today. 1991 Dec;12(12):429–431. doi: 10.1016/0167-5699(91)90013-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S., Kang S. H., Lee H. W., Trapani J. A., Dupont B., Yang S. Y. Isolation and expression of a cDNA clone encoding HLA-Cw6: unique characteristics of HLA-C encoded gene products. Immunogenetics. 1989;29(5):323–330. doi: 10.1007/BF00352842. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Rapp F. Enhanced capacity of DNA repair in human cytomegalovirus-infected cells. J Virol. 1981 Apr;38(1):164–172. doi: 10.1128/jvi.38.1.164-172.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Esber E., Saral R., Manischewitz J. F., Rogers J. L., Rook A. H., Santos G. W., Burns W. H. HLA-restricted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981 May;126(5):2036–2041. [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Weiland F., Münch K., Jonjic S., Lüske A., Koszinowski U. H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985 Aug;55(2):264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Quinnan G. V., Jr, Frederick W. J., Manischewitz J. F., Kirmani N., Dantzler T., Lee B. B., Currier C. B., Jr Importance of cytotoxic lymphocytes during cytomegalovirus infection in renal transplant recipients. Am J Med. 1984 Mar;76(3):385–392. doi: 10.1016/0002-9343(84)90655-7. [DOI] [PubMed] [Google Scholar]
- Saga S., Nagata K., Chen W. T., Yamada K. M. pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J Cell Biol. 1987 Jul;105(1):517–527. doi: 10.1083/jcb.105.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Sege K., Rask L., Peterson P. A. Role of beta2-microglobulin in the intracellular processing of HLA antigens. Biochemistry. 1981 Aug 4;20(16):4523–4530. doi: 10.1021/bi00519a003. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. B., Barber B. H., Flavell R. A., Allen H. Role of beta 2-microglobulin in the intracellular transport and surface expression of murine class I histocompatibility molecules. J Immunol. 1989 Apr 15;142(8):2796–2806. [PubMed] [Google Scholar]
- Woods C. M., Lazarides E. Degradation of unassembled alpha- and beta-spectrin by distinct intracellular pathways: regulation of spectrin topogenesis by beta-spectrin degradation. Cell. 1985 Apr;40(4):959–969. doi: 10.1016/0092-8674(85)90356-3. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Shimokata K., Mizuno S., Yamaguchi H., Nishiyama Y. Down-regulation of the surface expression of class I MHC antigens by human cytomegalovirus. Virology. 1993 Apr;193(2):727–736. doi: 10.1006/viro.1993.1181. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- van Dorp W. T., Jonges E., Bruggeman C. A., Daha M. R., van Es L. A., van Der Woude F. J. Direct induction of MHC class I, but not class II, expression on endothelial cells by cytomegalovirus infection. Transplantation. 1989 Sep;48(3):469–472. doi: 10.1097/00007890-198909000-00024. [DOI] [PubMed] [Google Scholar]