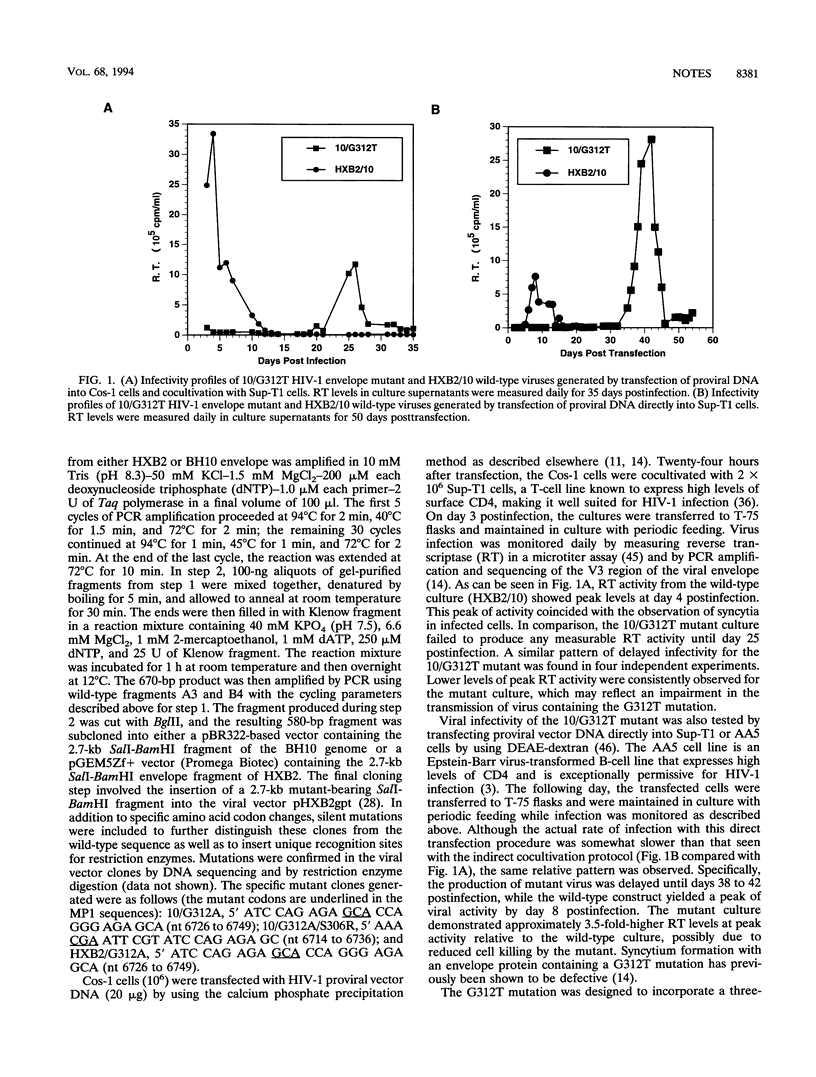
Abstract
Proviral clones of human immunodeficiency virus type 1 which contained single amino acid changes in the envelope V3 region were constructed. PCR amplification of Sup-T1 T cells transfected with one such mutant, G312T, revealed low levels of virus that resulted in the generation of a revertant virus, in which an alanine replaced the threonine residue at amino acid 312. The revertant virus (rA312) was fully infectious in Sup-T1 cells but lacked the ability to infect AA5 cells. The presence of a second mutation in a subsequent revertant virus (rR306), in which arginine was substituted for serine at amino acid 306 within the V3 loop, restored the ability of the mutated virus to infect AA5 cells. Our data highlight the importance of the V3 loop in defining virus tropism for specific cell types in culture and further suggest that a degree of interplay exists among V3 loop residues that helps maintain or control its biological function of the virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd M. T., Simpson G. R., Cann A. J., Johnson M. A., Weiss R. A. A single amino acid substitution in the V1 loop of human immunodeficiency virus type 1 gp120 alters cellular tropism. J Virol. 1993 Jun;67(6):3649–3652. doi: 10.1128/jvi.67.6.3649-3652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Bergeron L., Helseth E., Thali M., Repke H., Sodroski J. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J Virol. 1993 May;67(5):2747–2755. doi: 10.1128/jvi.67.5.2747-2755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee S., Leeds J. M., Matthews T. J., Weinhold K. J., Skinner M., Bolognesi D. P., Hershfield M. S. Phenotypic variation in the response to the human immunodeficiency virus among derivatives of the CEM T and WIL-2 B cell lines. J Exp Med. 1988 Aug 1;168(2):605–621. doi: 10.1084/jem.168.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- De Jong J. J., De Ronde A., Keulen W., Tersmette M., Goudsmit J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J Virol. 1992 Nov;66(11):6777–6780. doi: 10.1128/jvi.66.11.6777-6780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R. A., Groenink M., Kootstra N. A., Tersmette M., Huisman H. G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992 May;66(5):3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara J. B., Stura E. A., Stanfield R. L., Profy A. T., Wilson I. A. Crystal structure of the principal neutralization site of HIV-1. Science. 1994 Apr 1;264(5155):82–85. doi: 10.1126/science.7511253. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groenink M., Fouchier R. A., Broersen S., Baker C. H., Koot M., van't Wout A. B., Huisman H. G., Miedema F., Tersmette M., Schuitemaker H. Relation of phenotype evolution of HIV-1 to envelope V2 configuration. Science. 1993 Jun 4;260(5113):1513–1516. doi: 10.1126/science.8502996. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Ivanoff L. A., Dubay J. W., Morris J. F., Roberts S. J., Gutshall L., Sternberg E. J., Hunter E., Matthews T. J., Petteway S. R., Jr V3 loop region of the HIV-1 gp120 envelope protein is essential for virus infectivity. Virology. 1992 Apr;187(2):423–432. doi: 10.1016/0042-6822(92)90444-t. [DOI] [PubMed] [Google Scholar]
- Ivanoff L. A., Looney D. J., McDanal C., Morris J. F., Wong-Staal F., Langlois A. J., Petteway S. R., Jr, Matthews T. J. Alteration of HIV-1 infectivity and neutralization by a single amino acid replacement in the V3 loop domain. AIDS Res Hum Retroviruses. 1991 Jul;7(7):595–603. doi: 10.1089/aid.1991.7.595. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W. R., Matthews T. J., Ganfield M. C., Langlois A. J., Waselefsky D. M., Petteway S. R., Jr Antibodies from human immunodeficiency virus-infected individuals bind to a short amino acid sequence that elicits neutralizing antibodies in animals. AIDS Res Hum Retroviruses. 1989 Apr;5(2):173–182. doi: 10.1089/aid.1989.5.173. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A., Kinney-Thomas E., Hu S. L. Effects of anti-gp120 monoclonal antibodies on CD4 receptor binding by the env protein of human immunodeficiency virus type 1. J Virol. 1988 Oct;62(10):3695–3702. doi: 10.1128/jvi.62.10.3695-3702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- Milich L., Margolin B., Swanstrom R. V3 loop of the human immunodeficiency virus type 1 Env protein: interpreting sequence variability. J Virol. 1993 Sep;67(9):5623–5634. doi: 10.1128/jvi.67.9.5623-5634.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster T., Steindl F., Purtscher M., Trkola A., Klima A., Himmler G., Rüker F., Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993 Nov;67(11):6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K. A., Stearns S. M., Littman D. R. Analysis of mutations in the V3 domain of gp160 that affect fusion and infectivity. J Virol. 1992 Jan;66(1):524–533. doi: 10.1128/jvi.66.1.524-533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Fisher A., Jagodzinski L. L., Mitsuya H., Liou R. S., Gallo R. C., Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987 Spring;3(1):57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Orenstein J., Dimitrov D., Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992 Feb;186(2):712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell-line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Langlois A. J., McDanal C. B., McDougal J. S., Bolognesi D. P., Matthews T. J. Neutralizing antibodies to an immunodominant envelope sequence do not prevent gp120 binding to CD4. J Virol. 1988 Nov;62(11):4195–4200. doi: 10.1128/jvi.62.11.4195-4200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. D., Valenzuela A., Vigna J. L., Aalbers K., Lutz C. T. Unwanted mutations in PCR mutagenesis: avoiding the predictable. PCR Methods Appl. 1993 Feb;2(3):253–257. doi: 10.1101/gr.2.3.253. [DOI] [PubMed] [Google Scholar]
- Smith S. D., Shatsky M., Cohen P. S., Warnke R., Link M. P., Glader B. E. Monoclonal antibody and enzymatic profiles of human malignant T-lymphoid cells and derived cell lines. Cancer Res. 1984 Dec;44(12 Pt 1):5657–5660. [PubMed] [Google Scholar]
- Stamatatos L., Cheng-Mayer C. Evidence that the structural conformation of envelope gp120 affects human immunodeficiency virus type 1 infectivity, host range, and syncytium-forming ability. J Virol. 1993 Sep;67(9):5635–5639. doi: 10.1128/jvi.67.9.5635-5639.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N. C., Ho D. D., Sun C. R., Liou R. S., Gordon W., Fung M. S., Li X. L., Ting R. C., Lee T. H., Chang N. T. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3579–3585. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Akutsu M., Murayama K., Shimizu N., Hoshino H. Host range mutant of human immunodeficiency virus type 1: modification of cell tropism by a single point mutation at the neutralization epitope in the env gene. J Virol. 1991 Apr;65(4):1710–1718. doi: 10.1128/jvi.65.4.1710-1718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Trowbridge D. B., Epstein L. G., Blumberg B. M., Li Y., Hahn B. H., Shaw G. M., Price R. W., Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992 Apr;66(4):2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C., Oas T., McDanal C., Bolognesi D., Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Ross E. K., Buckler-White A. J., Theodore T. S., Martin M. A. Functional interaction of constant and variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3595–3600. doi: 10.1128/jvi.63.9.3595-3600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Yuan X., Matsuda Z., Lee T. H., Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992 Aug;66(8):4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J. J., Goudsmit J., Keulen W., Klaver B., Krone W., Tersmette M., de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992 Feb;66(2):757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


