Abstract
Adaptive behavior in autism is highly variable and strongly related to prognosis. This study explored family history as a potential source of variability in adaptive behavior in autism. Participants included 77 individuals (mean age=18) with average or better intellectual ability and autism. Parents completed the Family History Interview about the presence of broader autism phenotype symptoms and major psychiatric disorders in first degree relatives. Adaptive behavior was assessed via the Vineland Adaptive Behavior Scales (VABS). Based on family history variables, age, and intelligence quotient (IQ), 87% of participants were correctly classified as having impaired or average VABS scores. Family history of depression and shyness accounted for the most variance in VABS scores, and they had the greatest influence on VABS Socialization scores in particular. Possible underlying mechanisms include genetics, psychosocial factors, and social resources. This study provides initial evidence of the importance of family history to adaptive behavior in autism and has implications for genetics and treatment.
Keywords: Autism, Adaptive Behavior, Family History, Vineland Adaptive Behavior Scales, Broader Autism Phenotype
Introduction
Autism is a pervasive developmental disorder (PDD) defined by impairment in reciprocal social interaction, delayed and/or stereotyped communication, and restricted or repetitive behaviors and interests (American Psychiatric Association, 2000). PDDs are present in as many as 1 in 150 individuals (Centers for Disease Control, n.d.). Conceptualization of autism has dramatically changed over the past 20 years and it is now well-recognized that there is enormous variability in the expression of autism (Szatmari et al. 2002). Whereas it was previously believed that 70% or more of individuals with autism also had mental retardation (Rapin 1991), more recent estimates suggest that nearly half of individuals with autism may have an average or better intelligence quotient (IQ; Chakrabarti and Fombonne 2005). Another aspect of autism that makes it heterogeneous is that there is a wide range in terms of types and severity of symptoms (Rapin 1991; Rutter and Schopler 1987). This extreme variability has complicated the field’s understanding of how underlying brain mechanisms, genetics, environmental, and developmental factors interact in the etiology of autism.
Variability in autism is often quantified in terms of level of adaptive behavior skills, which are a multidimensional set of skills that provide an index of how an individual is able to function in the environment (Oswald and DiSalvo 2003). The vast majority of studies indicate that adaptive behavior skills in autism are lower than what would be expected for a given IQ and lower than IQ-matched controls (Bolte and Poutska 2002; Fenton et al. 2001). In general, the difference between IQ and adaptive behavior is greatest in samples of individuals with autism who are verbal and have average or better IQs (commonly referred to as high-functioning autism [HFA] e.g. Boltë and Poustka 2002; Liss et al. 2001). Most of the evidence suggests that adaptive behavior deficits tend to become more pronounced with increasing age (Fenton et al. 2001; Klin et al. 2007), though this research has predominantly focused only on children and adolescents.
Scores on the Vineland Adaptive Behavior Scale (VABS), the most widely used measure of adaptive behavior in autism, can range from four standard deviations below the mean to more than two standard deviations above the mean in populations of autism both with and without comorbid mental retardation (e.g. Klin et al. 2007; MacLean et al. 1999). The importance of adaptive behavior variability in autism is underscored by its strong contribution to prognosis (Gillham et al. 2003). Identifying sources of variability in adaptive behavior is critical to obtaining a more complete picture of development in autism as well as identification of treatment targets.
The first studies that attempted to identify developmental factors influencing adaptive behavior variability in autism produced initial evidence of a familial influence on VABS scores among siblings with autism spectrum disorders (Goin-Kochel et al. 2008; MacLean et al. 1999; Szatmari et al. 1996). Importantly, each study found significant intra-class correlations in support of reduced familial variance for VABS scores (Goin-Kochel et al. 2008; MacLean et al. 1999; Szatmari et al. 1996). This research suggests that some aspect of families may influence VABS variability, though the specific mechanism of influence is unclear.
Psychiatric family history is one important aspect of family functioning. Studies support the presence of related but milder characteristics, known as the Broader Autism Phenotype (BAP), in family members of individuals with autism (Piven 1999). Specifically, rates of BAP in families have varied from 12 to 50% across studies (e.g. Pickles et al. 2000; Piven and Palmer 1999). There are extremely high rates of psychiatric disorders, particularly affective (mood and anxiety) disorders, in both first degree relatives and extended family members of individuals with autism. Both BAP symptoms and affective disorders are present in families of children with autism at rates significantly higher than both the general population and families of children with other developmental disabilities (Bolton et al. 1998; Piven et al. 1991). The impact of such family history factors on proband vulnerability to autism or presentation is not yet entirely clear.
A relationship between family history factors and the child’s adaptive functioning is plausible given evidence of familial resemblance for VABS scores in affected siblings. The primary aim of this study was to clarify whether adaptive behavior in individuals with autism is related to family history characteristics of first degree relatives, such as depression or BAP symptoms. It was expected that family history information would account for a significant amount of variance in adaptive behavior in probands with HFA. This study focused on higher-functioning populations given more pronounced gaps between IQ and adaptive behavior in HFA and the importance of identifying factors to better understand why higher IQ does not necessarily translate into higher adaptive skills.
Methods
Participants
Participants in this study included 77 individuals with HFA (referred to as the proband participant) and at least one biological parent. Family history information was gathered on the first degree relatives of the individual with HFA. Demographic data are provided in Table 1. A wide age range was included (8–39 years old) in order to be able to explore and account for age effects in a wider age range than previous studies. All participants had Full Scale and Verbal IQ scores above 70 (i.e. did not have mental retardation) based on the appropriate Wechsler intelligence scale for the participant’s age. All participants spoke in complete sentences. Previous studies that included individuals with mental retardation found that family history patterns do not differ based on proband IQ (Starr et al. 2001). Potential subjects were excluded from the current study if they had associated neurologic, genetic, infectious, or metabolic disorders, such as tuberous sclerosis, fragile-X syndrome, or fetal cytomegalovirus infection.
Table 1.
Demographic and descriptive data
| Mean (SD) | Range | |
|---|---|---|
| Age in years | 18.08 (7.10) | 8–39 |
| Education (years)—Mom | 14.46 (2.71) | 3–22 |
| Education (years)—Dad | 15.45 (3.02) | 10–26 |
| SESa | 3.36 (1.53) | 1–7 |
| Verbal IQ | 105.87 (13.44) | 73–128 |
| Performance IQ | 105.66 (15.90) | 67–137 |
| Full scale IQ | 106.49 (13.99) | 74–135 |
| Percent male | 90.9% | |
| VABS adaptive behavior composite SS | 82.29 (21.77) | 27–118 |
| VABS communication SS | 84.96 (22.08) | 32–130 |
| VABS daily living SS | 91.14 (24.55) | 19–153 |
| VABS socialization SS | 80.36 (25.51) | 35–124 |
SES = socio-economic status, VABS = Vineland Adaptive Behavior Scales, SS = Standard Score (mean=100; SD=15)
SES was determined based on methods from Hollingshead (1957). This average reflects middle-class status (e.g. administrative personnel, small business owners)
All proband participants met criteria for autism on the Autism Diagnostic Observation Schedule (ADOS: Lord et al. 2000) for the Reciprocal Social Interaction, Communication, and Total algorithm scores. In addition, all participants met cutoffs for autism on the Autism Diagnostic Interview–Revised (ADI-R; Lord et al. 1994) for Reciprocal Social Interaction, Communication, and Restricted, Repetitive, and Stereotyped Behaviors and had abnormal development before 3 years of age. The diagnosis of autism established on the basis of the ADI-R and ADOS was verified by expert opinion based on accepted clinical descriptions of HFA (Minshew 1996; Minshew and Payton 1988; Rapin 1991; Rutter and Schopler 1987). A participant with ADI-R and ADOS scores above the cutoffs for autism could be ruled out on the basis of expert opinion, but expert opinion could not override ADI-R or ADOS scores that fell below the cutoff. The participants with autism were community volunteers recruited through advertisements in newsletters, postings on autism-related websites, and presentations for parents and professionals. The sample was based on consecutive referrals that met the inclusion criteria.
The University of Pittsburgh Institutional Review Board approved this study. Procedures were fully explained to all participants and to their parents or guardians. Written informed consent and assent was obtained from parents and children, respectively. All participants were recruited through the Subject Core of the University of Pittsburgh Collaborative Program of Excellence in Autism funded by the National Institute of Child Health and Human Development (PI: Minshew).
Measures
Vineland Adaptive Behavior Scales (VABS)
The VABS Survey form was administered to parents as a measure of how many age-appropriate, socially adaptive behaviors a child exhibits. It is a well-recognized instrument, with demonstrable reliability and validity both for individuals who are typically developing and those with disabilities (Sparrow et al. 1984). It is also the preeminent measure for the assessment of adaptive functioning in children with autism (Newsom and Hovanitz 1997). Three VABS skill domains were used in this study: Communication (receptive, expressive, and written language skills), Daily Living skills (personal self-care, domestic, and community living skills), and Socialization (interpersonal, play or leisure, and coping skills). The VABS provides standard scores (m=100, SD=15) and higher scores indicate better functioning.
Family History Interview for Developmental Disorders of Cognition and Social Functioning (FHI)
At least one informant (either the mother or father of the proband) was interviewed with the FHI, which was designed to provide standardized data on developmental disorders, social impairments, cognitive deficits, and psychiatric disorders in family members of individuals with PDDs (Rutter and Folstein 1995). It was developed and revised over 8 years. It uses an investigator-based approach that involves structured, operationalized coding. A coding of ‘2’ means that the precise criteria as specified in the schedule have been met; a coding of ‘1’ for ‘probable’ is used when the sufficient detail is unavailable to say that the criteria are certainly met, but it is highly likely; and, coding of ‘0” refers to normal functioning, mild abnormalities of the type specified but below the severity threshold, and abnormalities in the general domain (e.g. social relationships) but not of the type specified. The FHI has been widely used in autism research (e.g. Bolton et al. 1994; Starr et al. 2001) and has demonstrated validity (Fombonne et al. 1997; Rutter and Folstein 1995).
Analysis Plan
Descriptive analyses were conducted to characterize variability in the data. Outliers were identified. Outliers over three standard deviations from the mean were transformed to a score of exactly three standard deviations over the mean in order to retain the participants’ extreme status without having an undue influence on the conclusions (Tabachnick and Fidell 2006). To discern patterns in participant VABS scores, we employed Cluster Analysis which is an exploratory tool designed to reveal natural groupings within a dataset that would otherwise not be apparent (Aldenderfer and Blashfield 1984; Borden and Barnett 1987). Cluster analysis has produced useful taxonomies in health and medical research when a priori groupings are unknown (e.g. Hartigan 1975). Cluster analysis works by using distance and similarity measures to develop subgroups within the data that minimize within-group variance and maximize between-group variance. Cluster analyses were based on log likelihood distance measure and Schwarz’s Bayesian Criterion. Finally, we computed frequencies of family history variables.
For analyses related to this study’s primary aim, several methods were employed to reduce the possibility of Type I error, given the large number of family history variables. First, overall rates of family history variables by family were utilized (e.g. the highest level of depression in the family) rather than analyzing the presence of a disorder by family member. Second, only those family history variables endorsed as probably or definitely present in at least 10% of the sample (8 or more families) were included. This resulted in the use of the most prevalent symptoms/disorders only, reduced the overall number of variables and tests, and reduced the likelihood of outliers (e.g. families with a relatively rare symptom or disorder) skewing the results. Finally, age, IQ, and family history variables present in 10% or more of the population were all entered into a hierarchical logistic regression to predict proband VABS clusters. Follow-up hierarchical linear regression analyses were conducted to predict VABS domain scores from only the family history variables that were significant in the previous analysis, controlling for age and IQ.
Results
Descriptives
As can be seen in Table 1, there was great variability in proband VABS scores, with standard deviations over 21 and standard scores ranging from 19 to 162 (VABS standardization mean=100). Paired samples t-tests indicated that each VABS domain score was significantly lower than Full Scale, Verbal, and Performance IQs (p=0.000 for each comparison, specific results available upon request). Two-step cluster analysis of VABS domain scores indicated that participant scores were best explained by two clusters. The first cluster included 46% (n=32) of the sample; means for this cluster were average in each domain (Communication SS mean=100, Daily Living SS Mean= 106, and Socialization SS mean=103). The second cluster included 54% (n=38) of the sample; means for this cluster indicated impaired functioning in all three domains, with the lowest functioning in Socialization (Communication SS mean=78, Daily Living SS Mean=72, and Socialization SS mean=63). Therefore, there were two distinct clusters of adaptive functioning in this large sample of individuals with HFA—slightly less than half of the sample had average functioning in all domains and slightly more than half the sample had impaired adaptive functioning in all three domains.
Table 2 shows family history rates for all psychiatric disorders and BAP symptoms. Psychiatric disorders were present in at least one 1st degree relative at rates ranging from 3% to 33% (e.g. OCD in 14% of families; depression in 33% of families). The most prevalent issues in rank order were (1) depression, (2) worry, (3) anxiety, and (4) hyperactivity. Broader autism phenotype symptoms were highly prevalent in families, ranging from nearly 50% of families having a family member with extreme shyness to 4% having a family member with mental retardation.
Table 2.
Family history rates for presence of symptoms/disorders in any first degree relative
| Variable | Percent probable (n) | Percent definite (n) | Total percent |
|---|---|---|---|
| Depression | 20.8 (16) | 13.0 (10) | 33.8 |
| Anxious worrying | 18.2 (14) | 13.0 (10) | 31.2 |
| Anxiety disorders (not including phobias) | 15.6 (12) | 10.4 (8) | 26.0 |
| Hyperactivity | 13.0 (10) | 9.1 (7) | 22.1 |
| Obsessive-compulsive disorder | 7.8 (6) | 3.9 (3) | 11.8 |
| Alcohol abuse | 5.2 (4) | 3.9 (3) | 9.1 |
| Conduct disorder | 5.2 (4) | 3.9 (3) | 9.1 |
| Antisocial personality disorder | 5.2 (4) | 3.9 (3) | 9.1 |
| Drug abuse | 3.9 (3) | 1.3 (1) | 5.2 |
| Bipolar disorder | 1.3 (1) | 1.3 (1) | 2.6 |
| Shyness (to a degree that leads to withdrawal) | 28.6 (22) | 16.9 (13) | 45.5 |
| Oversensitivity to/misinterprets social comments | 20.8 (16) | 14.3 (11) | 35.1 |
| Impaired friendships/difficulty making friends | 18.2 (14) | 3.9 (3) | 22.1 |
| Rigid or perfectionistic/bothered by change | 14.3 (11) | 6.5 (5) | 20.8 |
| Circumscribed and unusually intense interests | 13.0 (10) | 2.6 (2) | 15.6 |
| Poor conversation skills | 10.4 (8) | 3.9 (3) | 14.1 |
| Autistic-like social dysfunction and isolation | 13.0 (10) | 0.0 (0) | 13.0 |
| Unusual beliefs | 9.1 (7) | 1.3 (1) | 10.4 |
| Lack of affection | 9.1 (7) | 1.3 (1) | 10.4 |
| Language delay | 5.2 (4) | 3.9 (3) | 9.1 |
| Impaired social play | 6.5 (5) | 1.3 (1) | 7.8 |
| Trouble with jobs because of social behavior | 5.2 (4) | 1.3 (1) | 6.5 |
| Mental retardation | 3.9 (3) | 1.3 (1) | 4.2 |
Relationship Between FHI and VABS
A hierarchical logistic regression was performed to predict VABS clusters. Age and IQ were entered in step one, followed by the family history variables present in at least 10% of the sample. The overall model was significant χ2 (15)=63.79, p=0.000 and accurately predicted VABS cluster membership for 87% of participants (see Table 3). Significant predictors included: Age (p=0.025), family history of autistic-like social dysfunction (p=0.048), family history of shyness (p=0.020), and family history of depression (p=0.015).
Table 3.
Vineland adaptive behavior clusters predicted by age, IQ, and family history variables
| Predicted cluster
|
|||||
|---|---|---|---|---|---|
| Cluster 1—all average | Cluster 2—impaired | Percent correct | |||
| Actual cluster | Cluster 1 | 31 | 27 | 4 | 87.5 |
| Cluster 2 | 39 | 5 | 34 | 90.0 | |
| Overall | 70 | 32 | 38 | 87.1 | |
Cells in bold refer to correctly identified participants
As follow-up analyses, separate hierarchical linear regressions were conducted to predict each VABS domain from the family history variables that were significant in the above analysis (autistic-like social dysfunction, shyness, and depression), controlling for age and IQ. Results indicated differing patterns of significant predictors for each of the three domains (see Table 4). Only older age and lower IQ were related to lower Communication scores. Lower Daily Living scores were predicted by older age, lower IQ, and a family history of depression. Familial depression accounted for nearly half of the explained variance in Daily Living above and beyond age and IQ. Finally, Socialization scores were significantly predicted by the presence of family history of depression and shyness only.
Table 4.
Results of linear regression analyses to predict vineland adaptive behavior domain scores
| Domain | Final model
|
Change statisticsa |
Final model independent variable t-scores (p-value)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| F (p-value) | R2 | F (p-value) | R2 | Age | IQ | Social dysfunction | Depression | Shyness | |
| Daily living | 5.73 (0.000)* | 0.30 | 4.43 (0.007)* | 0.14 | 2.07 (0.042)** | 1.99 (0.051)b | 0.28 (0.784) | −2.90 (0.005)* | −1.56 (0.124) |
| Communication | 3.97 (0.003)* | 0.23 | 2.04 (0.117) | 0.07 | −1.93 (0.058)b | 2.67 (0.010)** | 1.34 (0.184) | −1.42 (0.160) | −1.76 (0.082) |
| Socialization | 5.32 (0.000)* | 0.28 | 5.98 (0.001)* | 0.19 | 0.83 (0.411) | 1.42 (0.160) | 1.46 (0.150) | −2.77 (0.007)* | −2.80 (0.007)* |
Significant at p<0.01;
Significant at p<0.05
Change statistics refer to the addition of family history variables in Step 2 above and beyond age and IQ
Nearly significant
To facilitate interpretation of the above findings, the linear regression analyses were duplicated using only parental family history variables (e.g. excluding siblings). The results were virtually identical, with the same significant predictors, and magnitude and direction of effects (specific results available upon request). In addition, analyses were conducted to rule out the possibility that the above significant findings were an artifact of family history predicting symptom severity, which may in turn predict adaptive behavior. In an effort to reduce multiple testing, this extra analysis only focused on the family history factors that were significantly related to adaptive behavior. Specifically, correlations were run between ADOS social totals, ADI social totals, ADOS communication totals, and ADI communication totals (all used as indicators of symptom severity) and parental history of depression, social dysfunction, and shyness. None of the correlations were significant (p>0.05 in all cases, p values ranged from 0.17–0.98). The magnitude of correlations was very small, ranging from 0.04 to −0.14.
Discussion
This study explored whether adaptive behavior in individuals with autism is related to the family history characteristics of their first degree relatives. The results of this study confirmed extremely high variability in adaptive behavior in autism, even among a more homogeneous and higher-functioning sample of individuals who were verbally fluent and without mental retardation. These findings further emphasize the importance of identifying salient developmental influences on adaptive behavior functioning in autism. Our results replicated earlier findings that adaptive behavior deficits in the areas of daily living and communication become more apparent with age (e.g. Klin et al. 2007) and provide initial evidence that this pattern extends into adulthood. Our results also confirm that the difference between IQ and adaptive behavior is significant even in high-functioning samples (Boltë and Poustka 2002; Klin et al. 2007; Liss et al. 2001). Moreover, our results suggest that IQ may not be as important a predictor of adaptive behavior as family history variables. Specifically, 86% of participants were correctly identified as falling into either an impaired or average VABS cluster based on family history variables, age, and IQ, but only family history variables and age were significant predictors. These results are consistent with research suggesting a familial influence on adaptive behavior in autism (e.g. Goin-Kochel et al. 2008). This is the first study, to our knowledge, to test and demonstrate a relationship between family history and adaptive behavior in autism.
Follow-up analyses revealed different significant predictors for the Communication, Daily Living, and Socialization domain scores on the VABS domain. The only domain not significantly predicted by family history variables was Communication, which was mostly related to IQ and was also influenced by age. These findings are consistent with earlier research indicating that the adaptive behavior domain most linked to IQ in HFA is Communication (Klin et al. 2007). IQ and age also explained a significant amount of variance in VABS Daily Living scores, but a nearly equal amount of variance was explained by depression in family members. The relationship between family history variables and adaptive behavior was most prominent for Socialization, in that the only significant predictors were depression and shyness in family members. This result supports earlier findings that the rate of growth in socialization is not related to initial IQ (Freeman et al. 1999) and points to family history variables as a more important influence on social functioning. It is interesting that depression was the most influential family variable, given the wealth of literature from typical child development implicating parental depression in negative childhood adjustment (for review see Downey and Coyne 1990; Goodman and Gotlieb 1999). Although the majority of this literature has focused on child outcomes such as psychiatric disorders and behavioral problems, adapting these models to explain the impact of family history on adaptive behavior in autism seems warranted.
There are many potential mechanisms that may underlie the link between family history factors and adaptive behavior in HFA. Our results indicated that the key family history factors found to influence adaptive behavior (depression, social dysfunction, and shyness) were not correlated with autistic symptom severity, suggesting that the family history-adaptive behavior relationship is not mediated by symptom severity. Figure 1, based on models of the influence of maternal depression on childhood adjustment (e.g. Elgar et al. 2004; Goodman and Gotlieb 1999), demonstrates some other potential mediators. The model has been modified to include specific pathways that may be particularly pertinent to explaining the link between familial depression and socialization skills of individuals with autism. First, there may be a unidirectional genetic susceptibility to poor socialization skills conferred by familial depression. Second, genetics may indirectly impact child socialization by increasing risk for comorbidity with depression and anxiety. This path is likely for two reasons: (a) Successful emotion regulation is critical for the maintenance of an optimal state of arousal to meet environmental demands in daily life and attend to appropriate social information (Laurent and Rubin 2004), and (b) Evidence suggests extremely high rates of comorbid anxiety and depression in autism (Leyfer et al. 2006). Third, psychosocial factors (e.g. modeling, parent–child interactions, general family functioning, and environmental stress) may mediate the relationship between familial depression and child socialization. The need for the family members to make more of an effort in social interactions with the proband is increased given the tendency for individuals with autism to have a greater level of disengagement, yet unlikely to occur as frequently if the family member is depressed. This pathway may also work in the reverse such that lower child socialization skills could lead to worse family functioning, more limited positive interaction, and greater environmental stress, which could increase familial depression and shyness. Finally, social resources such as general economic strain and availability of treatments are likely to influence both child socialization and familial depression. Furthermore, depressed family members are less likely to utilize the resources that are available, which could lead to poorer child socialization skills.
Fig. 1.
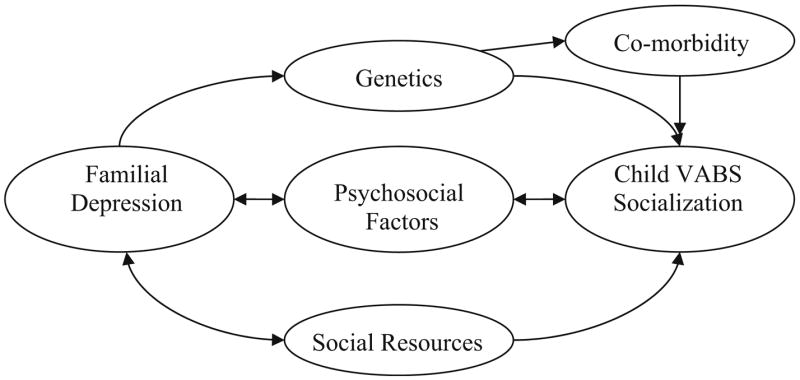
Hypothesized pathways between family history of depression and the Vineland Adaptive Behavior Scale (VABS) socialization domain score of the family member with autism
Several of the above mentioned hypothesized pathways may also explain the relationship between familial shyness and proband socialization. The one primary exception with applying the above model to familial shyness is that pathways are less likely to play a role from proband socialization to familial excessive shyness. The likelihood of a more unidirectional pathway from familial shyness to proband socialization even further implicates the potential role of genetics. This hypothesis is supported by findings indicating that a “social motivation” variable from a newly developed BAP measure, which is a very similar construct to the excessive shyness variable measured by the FHI, is the most heritable BAP trait (Dawson et al. 2007; Sung et al. 2005). Sung et al. (2005) concluded that social motivation may be a key variable to stratify samples for gene mapping given its higher heritability overall. Our findings extend the implications of this previous research, by suggesting that familial shyness/decreased social motivation may pose a specific risk for the degree of proband impairment in social functioning. Identifying such phenotypes with potential genetic relevance to variability in autism presentation is critical, given the acknowledged complexity and heterogeneity of the genetic pathway to autism (Bacchelli and Maestrini 2006) and the need for stratification techniques that are likely to produce replicable genetic findings.
Potential limitations of this study should be considered when interpreting results. Given the large number of family history variables and need to limit the number of tests, we were unable to specifically explore Vineland subdomains (e.g. expressive language, receptive language) and the impact of family history variables by family member (e.g. depression in mothers, depression in sisters, etc). In addition, family history data was gathered from one family member whereas it would be preferable to directly interview each member with a standardized psychiatric interview. However, the FHI has been validated and has been widely and successfully used in previous autism research (e.g. Bolton et al. 1994; Starr et al. 2001). We did not have data available on potential influences on VABS domains such as the proband’s history of treatment and whether or not he/she had a comorbid psychiatric disorder. Our study employed a large age range, which we controlled for statistically. Although this could be perceived as a weakness, it allowed us to explore the relationship between age and VABS into adulthood whereas previous research on this relationship has only focused on participants 18 years of age and younger. Our results may not generalize to populations with autism and mental retardation given our focus on HFA and future research is necessary to determine if patterns of association differ between individuals with autism and mental retardation and those without. Finally, we were not able to compare our data to controls to determine if the relationship between family history and VABS scores is unique to or stronger in autism, which should be explored in future studies due to important genetic implications.
Despite these limitations, this study provides initial and compelling evidence of the importance of family history to VABS scores of individuals with autism and has several significant implications. Family history is relevant to treatment approaches. Perhaps improvement in socialization and daily living skills in particular would be greater if treatment models began with assessment of family mental health and then more actively involved family members in treatment rather than focusing solely on increasing the skills of the child with autism. Further study is needed to clarify the mechanisms proposed in the above figure, but they also have implications for treatment if confirmed. For example, if the pathway between parental depression and proband socialization is mediated by psychosocial factors, interventions should target increasing parent–child interactions and modeling of prosocial behavior, and decreasing environmental and household stress. Similarly, if the pathway related to social resources is confirmed, perhaps families with depressed family members in particular would benefit from increased case management efforts and facilitation of finding and using available treatments.
There are also several potentially interesting directions for future research. It will be important for future research to measure and test the mediators we proposed in Fig. 1. Specifically, future studies will need to determine how much the family environment, familial genetic predisposition, or their interactions play a role in determining the adaptive functioning of the family member with autism. Future research should explore more specific patterns of association by including VABS subdomains and exploring family history variables by family member. It would also be interesting to explore the impact of mental health state in terms of the impact of current depression versus history of depression and the impact of recurrent depression. Finally, it would be informative to compare the findings of the impact of family history on VABS scores to the association between these variables in both typically developing and other developmental disability populations.
Acknowledgments
This manuscript was supported by NICHD HD35469 (PI Minshew), A National Institutes of Health Collaborative Program of Excellence in Autism Study.
Contributor Information
C. A. Mazefsky, Departments of Pediatrics and Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA
D. L. Williams, Department of Speech–Language Pathology, Duquesne University, Pittsburgh, PA, USA
N. J. Minshew, Departments of Psychiatry and Neurology, University of Pittsburgh, Pittsburgh, PA, USA
References
- Aldenderfer MS, Blashfield RK. Cluster analysis. Beverly Hills, CA: Sage; 1984. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Bacchelli E, Maestrini E. Autism spectrum disorders: Molecular genetic advances. American Journal of Medical Genetics Part C. 2006;142C:13–23. doi: 10.1002/ajmg.c.30078. [DOI] [PubMed] [Google Scholar]
- Boltë S, Poustka F. The relation between general cognitive level and adaptive behavior domains in individuals with autism with and without co-morbid mental retardation. Child Psychiatric and Human Development. 2002;33:165–172. doi: 10.1023/a:1020734325815. [DOI] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case-control family study of autism. Journal of Child Psychology and Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and other psychiatric disorders: Patterns of familial aggregation. Psychological Medicine. 1998;28:385–395. doi: 10.1017/s0033291797006004. [DOI] [PubMed] [Google Scholar]
- Borgen FH, Barnett DC. Applying cluster analysis in counseling psychology research. Journal of Counseling Psychology. 1987;34:456–468. [Google Scholar]
- Centers for Disease Control. Prevalence of the autism spectrum disorders in multiple areas of the United States, surveillance years 2000 and 2002. nd Retrieved July 18, 2007 from http://www.cdc.gov/od/oc/media/pressrel/2007/r070208.htm.
- Chakrabarti S, Fombonne E. Pervasive Developmental Disorders in preschool children: Confirmation of high prevalence. American Journal of Psychiatry. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- Dawson G, Estes A, Munson J, Schellenberg G, Bernier R, Abbott R. Quantitative assessment of autism symptom-related traits in probands and parents: Broader phenotype autism symptom scale. Journal of Autism and Developmental Disorders. 2007;37:523–536. doi: 10.1007/s10803-006-0182-2. [DOI] [PubMed] [Google Scholar]
- Downey G, Coyne J. Children of depressed parents: An integrative review. Psychological Bulletin. 1990;108:50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- Elgar FJ, McGrath PJ, Waschbusch DA, Stewart SH, Curtis LJ. Mutual influence on maternal depression and child adjustment problems. Clinical Psychology Review. 2004;24:441–459. doi: 10.1016/j.cpr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Fenton G, D’Ardia C, Valente D, Del Vecchio I, Fabrizi A, Bernabei P. Vineland adaptive behavior profiles in children with autism and moderate to severe developmental delay. Autism. 2001;7:269–287. doi: 10.1177/1362361303007003004. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Bolton P, Prior J, Jordan H, Rutter M. A family study of autism: Cognitive patterns and levels in parents and siblings. Journal of Child Psychology and Psychiatry. 1997;38:667–683. doi: 10.1111/j.1469-7610.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Freeman BJ, Del’Homme M, Guthrie D, Zhang F. Vineland adaptive behavior scale scores as a function of age and initial IQ in 210 autistic children. Journal of Autism and Developmental Disorders. 1999;5:379–384. doi: 10.1023/a:1023078827457. [DOI] [PubMed] [Google Scholar]
- Gillham JE, Carter AS, Volkmar FR, Sparrow SS. Toward a developmental operational definition of autism. In: Hertzig ME, Farber EA, editors. Annual progress in child psychiatry and child development: 2000–2001. New York, NY: Brunner-Routledge; 2003. pp. 363–381. [DOI] [PubMed] [Google Scholar]
- Goin-Kochel RP, Mazefsky CA, Riley BP. Level of functioning in autism spectrum disorders: Phenotypic congruence among affected siblings. Journal of Autism and Developmental Disorders. 2008 doi: 10.1007/s10803-007-0476-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. New Haven, CT: Yale University Press; 1957. [Google Scholar]
- Hartigan J. Clustering algorithms. New York: Wiley; 1975. [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher-functioning individuals with autism spectrum disorders: The Vineland and ADOS. Journal of Autism and Developmental Disorders. 2007;37:748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Laurent AC, Rubin E. Challenges in emotional regulation in Asperger syndrome and high-functioning autism. Topics in Language Disorders. 2004;24:286–297. [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Liss M, Harel B, Fein D, Allen D, Dunn M, Feinstein C, et al. Predictors and correlates of adaptive functioning in children with developmental disorders. Journal of Autism and Developmental Disorders. 2001;31:219–230. doi: 10.1023/a:1010707417274. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur AL. Autism diagnostic interview—revised: A revised version of the diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MacLean JE, Szatmari P, Jones MB, Bryson SE, Mahoney WJ, Bartolucci G, et al. Familial factors influence level of functioning in pervasive developmental disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:746–753. doi: 10.1097/00004583-199906000-00023. [DOI] [PubMed] [Google Scholar]
- Minshew NJ. Autism. In: Berg BO, editor. Principles of child neurology. New York: McGraw-Hill; 1996. pp. 1713–1730. [Google Scholar]
- Minshew NJ, Payton JB. New perspectives in autism, part I: The clinical spectrum of autism. Current Problems in Pediatrics. 1988;18:561–610. doi: 10.1016/0045-9380(88)90021-7. [DOI] [PubMed] [Google Scholar]
- Newsom C, Hovanitz CA. Autistic disorder. In: Mash EJ, Terdal LG, editors. Assessment of childhood disorders. 3. New York: Guilford; 1997. pp. 408–452. [Google Scholar]
- Oswald DP, DiSalvo CA. Adaptive behavior assessment. In: Ollendick TH, Schroeder CS, editors. The encyclopedia of pediatric and clinical child psychology. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, Bailey A, et al. Variable expression of the autism broader phenotype: Findings from extended pedigrees. Journal of Child Psychology and Psychiatry. 2000;41:491–502. [PubMed] [Google Scholar]
- Piven J. Genetic liability for autism: The behavioural expression in relatives. International Review of Psychiatry. 1999;11:299–308. [Google Scholar]
- Piven J, Landa R, Gayle J, Cloud D, Chase G, Folstein S. Psychiatric disorders in the parents of autistic individuals. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:471–478. doi: 10.1097/00004583-199105000-00019. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. American Journal of Psychiatry. 1999;156:557–563. doi: 10.1176/ajp.156.4.557. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autistic children: Diagnosis and clinical features. Pediatrics. 1991;87:751–760. [PubMed] [Google Scholar]
- Rutter M, Folstein S. Family History Interview for Developmental Disorders of Cognition and Social Functioning. 1995 Unpublished interview. [Google Scholar]
- Rutter M, Schopler E. Autism and pervasive developmental disorders: Concepts and diagnostic issues. Journal of Autism and Developmental Disorders. 1987;17:159–186. doi: 10.1007/BF01495054. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Starr E, Berument SK, Pickles A, Tomlins M, Bailey A, Papanikolaou K, et al. A family genetic study of autism associated with profound mental retardation. Journal of Autism and Developmental Disorders. 2001;31:89–96. doi: 10.1023/a:1005669915105. [DOI] [PubMed] [Google Scholar]
- Sung YJ, Dawson G, Munson J, Estes A, Schellenberg GD, Wijsman EM. Genetic investigation of quantitative traits related to autism: Use of multivariate polygenic models with ascertainment adjustment. American Journal of Human Genetics. 2005;76:68–81. doi: 10.1086/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Jones MB, Holden J, Bryson S, Mahoney W, Tuff L, et al. High phenotypic correlations among siblings with autism and pervasive developmental disorders. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 1996;67:354–360. doi: 10.1002/(SICI)1096-8628(19960726)67:4<354::AID-AJMG7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Merette C, Bryson SE, Thivierge J, Roy M, Cayer M, et al. Quantifying dimensions in autism: A factor analytic study. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:467–474. doi: 10.1097/00004583-200204000-00020. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Needham Heights, MA: Allyn & Bacon; 2006. [Google Scholar]


