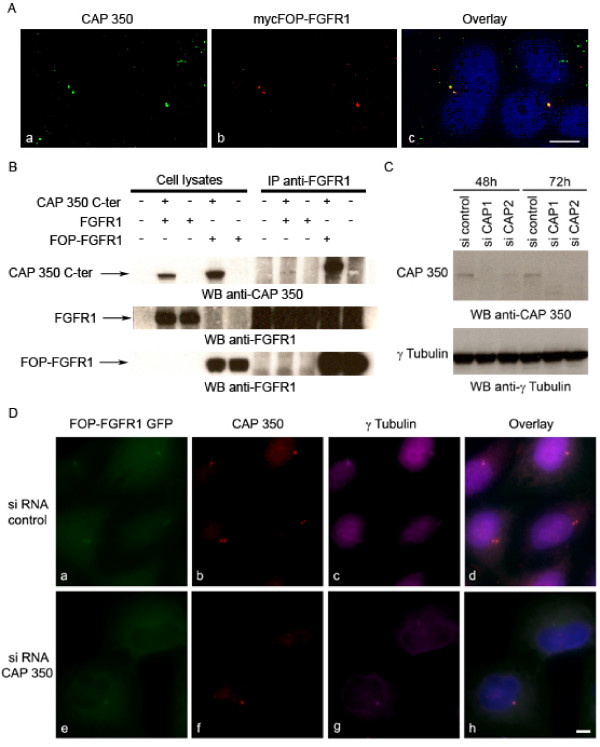
Figure 1.
FOP-FGFR1 interacts with CAP350 and CAP350 depletion prevents FOP-FGFR1 centrosomal localization. (A) FOP-FGFR1 colocalizes with endogenous CAP350. Immunofluorescence experiments are done on HeLa cells transiently transfected with mycFOP-FGFR1. Costaining using anti-CAP350 (green) and anti-myc (red) antibody reveals colocalization at the centrosome of CAP350 and mycFOP-FGFR1. Scale bar 5 μm. (B) FOP-FGFR1 interacts with CAP350 in lysates from Cos-1 cells transiently transfected with vectors expressing CAP350 C-terminus, and either FOP-FGFR1 or FGFR1. Immunoprecipitations are done with anti-FGFR1 antibody and bound CAP350 is revealed by western blotting using anti-CAP350 antibody. Anti-FGFR1 western blotting confirms the efficiency of the transfection. (C) Western blot with anti-CAP350 antibody shows reduced level of CAP350 in HeLa cell lysates after siRNA treatment for 48 h or 72 h with siCAP duplex as compared to sicontrol duplex. γ-tubulin is shown as a loading control. (D) Immunofluorescence of CAP350 depleted with siRNA CAP350 (bottom) and control (top) in FOP-FGFR1 GFP HeLa cells (a, b) and anti-CAP350 antibody (b, f) and γ-tubulin antibody (c, g). CAP350 depleted cells lack FOP-FGFR1. Scale bar 5 μm.