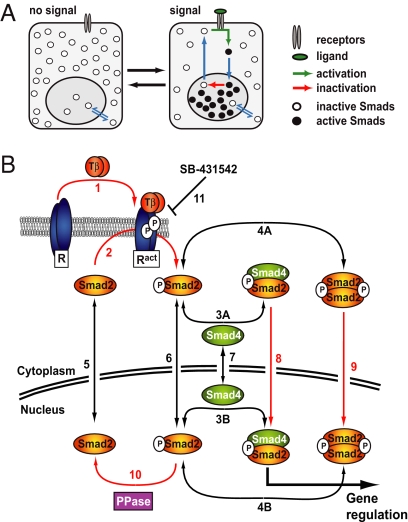
Fig. 1.
Smad nucleocytoplasmic dynamics. (A) A cartoon of Smad nucleocytoplasmic dynamics. Inactive Smads shuttle between the nucleus and cytoplasm. Receptor-activated Smads reside preferentially in the nucleus and accumulate there. Slow inactivation of active Smads in the nucleus, however, continuously releases inactivated Smads into the cytoplasm. In the presence of a signal, reactivation of cytoplasmic Smads dynamically maintains nuclear accumulation. As soon as receptor activity ceases, this mechanism restores the system back to the uninduced state. (B) Network topology used for model construction. Red arrows indicate irreversible reactions. Black arrows indicate reversible reactions. TGF-β binding converts inactive receptors (R) irreversibly into active receptors (Ract) with a rate constant kTGFβ (1). Active receptors irreversibly phosphorylate cytoplasmic Smad2 with a rate constant kphos (2). In both the nucleus and the cytoplasm, phospho-Smad2 forms heteromeric complexes with Smad4 (3A and 3B) and homomeric complexes (4A and 4B). These reversible reactions are described by the off-rate koff and the dissociation constant Kdiss. Smad2 (5), monomeric phospho-Smad2 (6), and Smad4 (7) shuttle reversibly between nucleus and cytoplasm with the rate constants kin and kex. Heteromeric complexes (8) and homomeric complexes (9) are imported into the nucleus with a rate constant kin times CIF (complex import factor), but cannot be exported. The Smad2 phosphatase (PPase) is nuclear and irreversibly dephosphorylates monomeric phospho-Smad2 with a rate constant kdephos (10). Finally, the receptor kinase is reversibly blocked by the inhibitor SB-431542 (11), which is described by the interaction's off-rate, koffSB, and dissociation constant, KdissSB. For further details see SI Text and Fig. S2.