Table 1.
Kinetic and equilibrium constant governing binding of BirA variants to biotin and bio-5`-AMPa
| Variant/ ligand | koff (s−1) | kon(M−1 s−1) | KD(M) |
| biotin | |||
| wt | 0.33 (±0.01) | 7.4 (±0.3) × 106 | 4.5 (±0.2) × 10−8 |
| G115S | 80 (±20) | 9 (±2) × 106 | 9 (±3) × 10−6 |
| R118G | 29 (±5) | 1.6 (±0.2) × 107 | 1.8 (±0.4) × 10−6 |
| R119W | 0.49 (±0.04) | 2.2 (±0.2) × 106 | 2.5 (±0.3) × 10−7 |
| bio-5`-AMP | |||
| wt | 0.00027 (±0.00003) | 6.0 (±0.6) × 106 | 4.5 (±0.7) × 10−11 |
| G115S | 0.84 (±0.03) | 7.0 (±0.7) × 106 | 1.2 (±0.1) × 10−7 |
| R118G | 0.119 (±0.006) | 6 (±1) × 106 | 2.0 (±0.3) × 10−8 |
| R119W | 0.00009 (±0.00001) | 4.9 (±0.2) × 106 | 1.8 (±0.2) × 10−11 |
a All parameters were previously reported in Kwon and Beckett (2000). The equilibrium dissociation constants (KD) were calculated from the measured unimolecular dissociation rate constants (koff) and bimolecular association rate constants (kon) using the equation 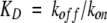 . Parameters were determined in Standard Buffer (10 mM TrisHCl, pH 7.50 ± 0.02, 200 mM KCl, 2.5 mM MgCl2 at 20.0 ± 0.1°C).
. Parameters were determined in Standard Buffer (10 mM TrisHCl, pH 7.50 ± 0.02, 200 mM KCl, 2.5 mM MgCl2 at 20.0 ± 0.1°C).
