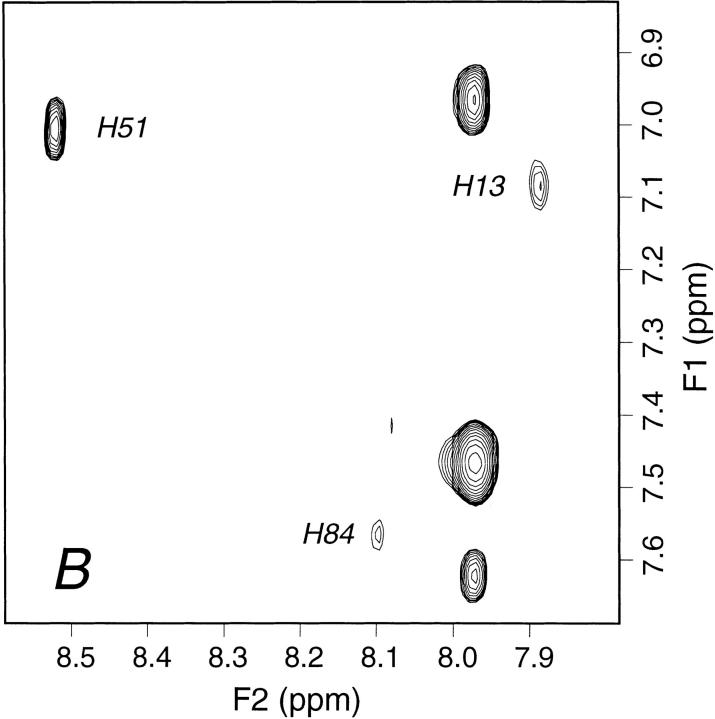
Fig. 7.
Aromatic region from 1H 2D TOCSY spectrum of 0.71 mM β2-m in 70 mM phosphate buffer, 100 mM NaCl at pH 6.6, T = 310 K, (A) in the absence and (B) in the presence of Cu2+ at a metal/protein concentration ratio of 3:10. Cu2+ titration experiments were performed by successive addition of 1 μL aliquots from a 35 mM CuCl2 solution. The histidine Hδ2–Hɛ1 cross-peak assignments are indicated. The complete bleaching of the connectivity arising from His 31 in the presence of Cu2+ is consistent with a specific reversible binding of the ion to the imidazole ring of the residue. The extent of resonance attenuation reflects an efficient averaging of the paramagnetic perturbation due to fast Cu2+ exchange rate, that is, faster than the transverse relaxation rates of His 31 aromatic hydrogens. Partial attenuation is also observed for the connectivity attributed to His 13, the next available binding site along the partial unfolding pathway; whereas, no effect is detectable for the other two histidine cross-peaks (His 51 and His 84) and also on increasing the metal ion/protein ratio up to 1:1. In addition to the imidazole ring connectivities, only the Hβ resonances of His 31 and His 13 are detectably attenuated by further Cu2+ addition (i.e., beyond ion/protein ratio of 0.3), due to spatial proximity of the paramagnetic species binding site.