Fig. 3.
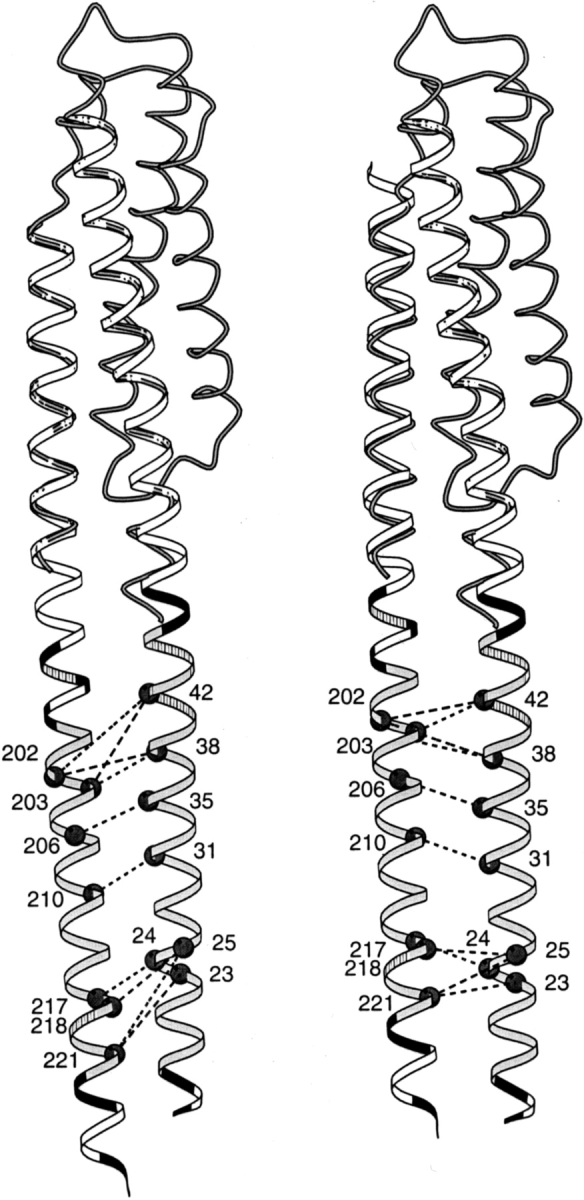
Positions of cysteine pairs with high propensity for cross-linking transmembrane helix 1 (TM1) and transmembrane helix 2 (TM2) of Trg. Initial (left) and revised (right) positions of TM2 relative to TM1 are diagrammed with cysteine pairs, showing high cross-linking propensities labeled with dark circles and joined by dashed lines. The cross-links shown are those in Lee et al. (1994), excluding one pair that showed very modest cross-linking on later quantification and four pairs created after that study that show cross-linking propensities within the range of the initial set (Beel and Hazelbauer 2001). The shaded coil shows the backbone structure of the monomer of the periplasmic domain (1LIH; Milburn et al. 1991) that was used to align the helices of the extended coiled coil template (ribbon). (Right) The sliding of α4/TM2 toward the periplasm has been emphasized by shifting only the coiled coil template, not the coil representing the original position of helix α4. The hydrophobic core of the transmembrane domain is shaded in light gray, positions of aromatic and amidated residues at the edges of the hydrophobic core are striped, and charged residues (including histidines) are black. This figure was made using MolScript (Kraulis 1991).
