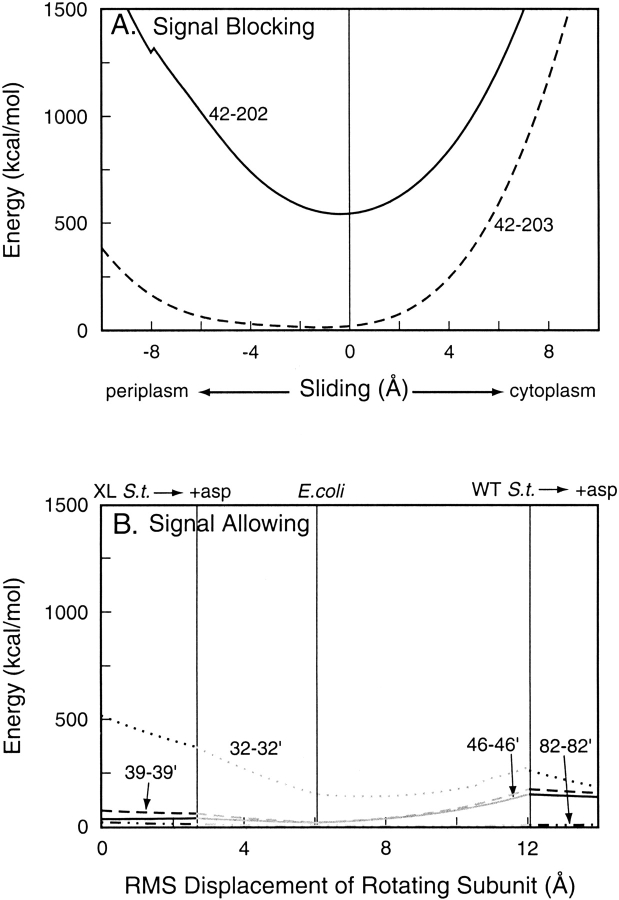
Fig. 5.
Energies of inter-helical disulfides in chemoreceptor Trg as a function of ligand-induced conformational changes. Values for potential energy of disulfide bonds in models of oxidatively cross-linked Trg periplasmic plus cytoplasmic domains were calculated at incremental steps of two ligand-induced conformational changes. (A) Energies of signal-blocking transmembrane helix 1 (TM1)–transmembrane helix 2 (TM2) disulfides between the indicated positions over the course of a sliding displacement of TM2 along its long axis. The kink in the 42–202 curve reflects a switch in dihedral angle from one conformation to another. (B) Energies of signal-allowing TM1-TM1` disulfides between the indicated positions over the course of rotation of one subunit from one to another orientation in the crystal structures of the periplasmic domain. The abscissa shows the total root mean square distance traveled by the moving subunit. Positions of the crystal structures are indicated along the top. XL S.t. indicates cross-linked Salmonella structure; WT S.t., wild-type Salmonella structure; and asp, aspartate.