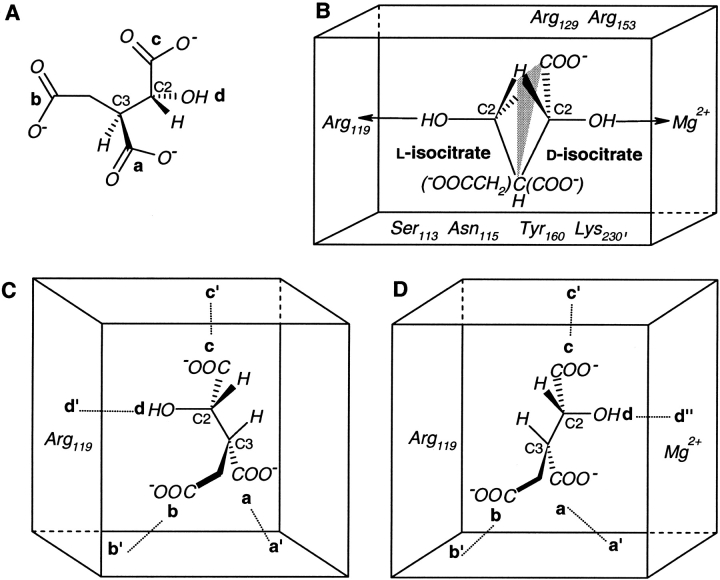
Fig. 7.
Isocitrate and its interactions with the enzyme isocitrate dehydrogenase (IDH). (A) Four locations in (2R,3S)-isocitrate distributed in a (2-2) configuration, a (—COO−) and b (—CH2COO−) on the C3 stereocenter along with c (—COO−) and d (—OH) on the C2 stereocenter. (B) Superposition of different interactions of d-isocitrate [(2R,3S)] and l-isocitrate [(2S,3R)] with IDH in the presence and absence of metal (Mg2+), respectively. Only the important enzyme residues in the substrate binding site are shown. A hypothetical mirror-plane is shown passing through H, COO−, and C3. (C) Schematic representation of (2S,3R)-isocitrate bound to metal-free IDH. a′ (Arg 153, Arg 119, Tyr 160, Lys 230′), b′ (Ser 113, Asn 115), and c′ (Arg 119, Arg 129, Arg 153) are enzyme residues that interact with substrate locations a, b, and c, respectively. d′ (Arg 119 and a water molecule) binds to the —OH group. (D) Substrate locations a, b, and c in (2R,3S)-isocitrate bind to metal-containing IDH at the same three enzyme sites a′, b′, and c′, but the —OH group binds to d" (Mg2+ and the enzyme residues bound to it).