Fig. 6.
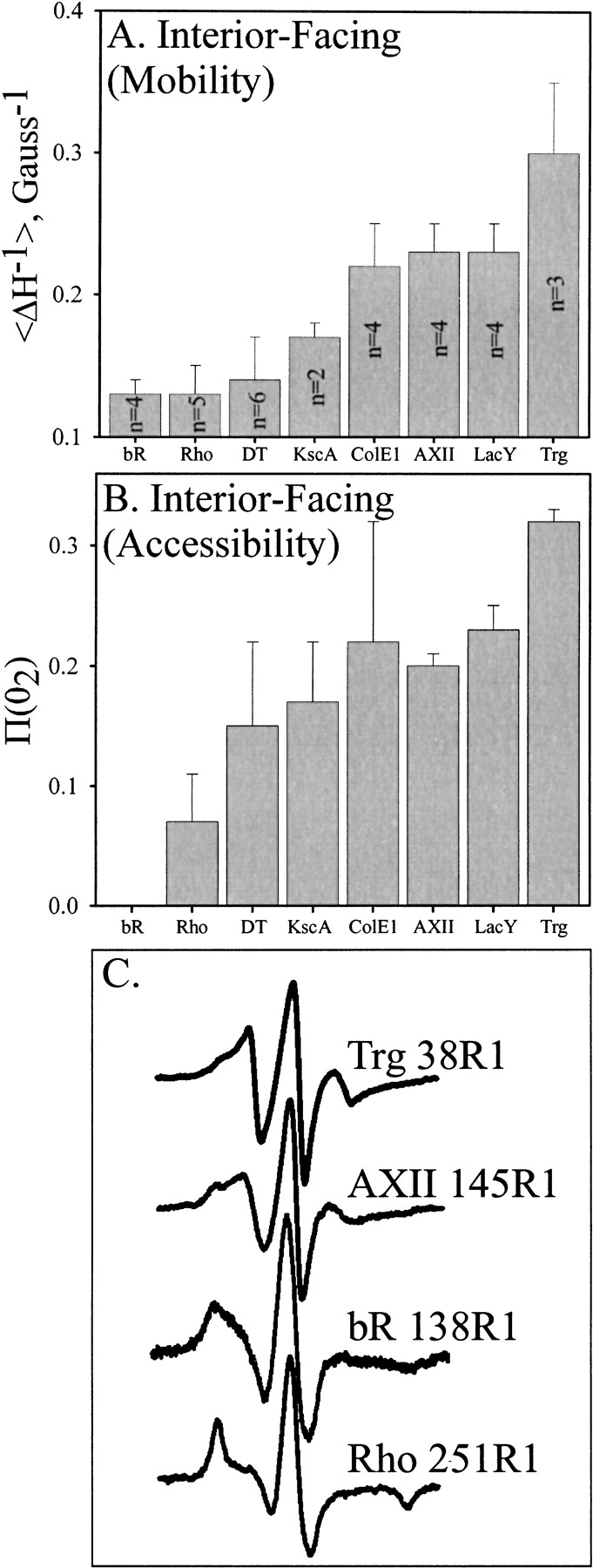
Mobility and accessibility of interior-facing R1 residues on transmembrane α-helices characterized by site-directed spin labeling. Average values for the mobility parameter Δ H−1 (A) or the accessibility parameter |gP(O2) (B) are shown for interior-facing helical positions of bacteriorhodopsin (bR) (Altenbach et al. 1990, 1994), rhodopsin (Rho) (Altenbach et al. 1996), diphtheria toxin T domain (DT) inserted into bilayers at low pH (Oh et al. 1996), Streptomyces lividans potassium channel (KscA) (Gross et al. 1999), colicin E1 (ColE1) inserted into bilayers at low pH (Salwinski and Hubbell 1999), annexin XII (AXII) inserted into membranes at low pH (Langen et al. 1998), lactose permease (LacY) (Voss et al. 1996), and TM1 of Trg (this study). For each protein, all the sites were along a single helix. The number of values averaged is indicated within the bar and was the same for panels A and B. Error bars represent the standard deviation from the mean. Representative spectra from positions used in the calculations for panel A are shown in panel C.
