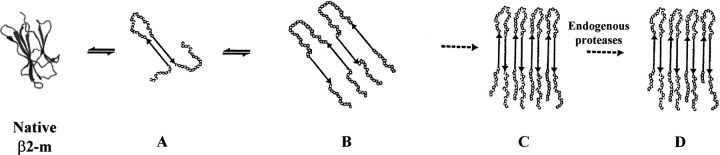
Fig. 6.
Possible fibrillogenic pathway of β2-m. Native β2-m undergoes conformational changes to generate the partly unfolded intermediate (A) characterized by an amyloidogenic conformation whose overall topologic structure resembles that of the truncated ΔN6β2-m form. The transient component (A) originates the accumulation of soluble aggregates (B), which, in turn, drives the entire equilibrium toward the formation of fibrils (C). When the extracellular proteases attempt to digest the insoluble material, the tightly packed structure of the fibril core prevents proteolysis and addresses protease action toward the amino-and carboxy-terminal ends (D).