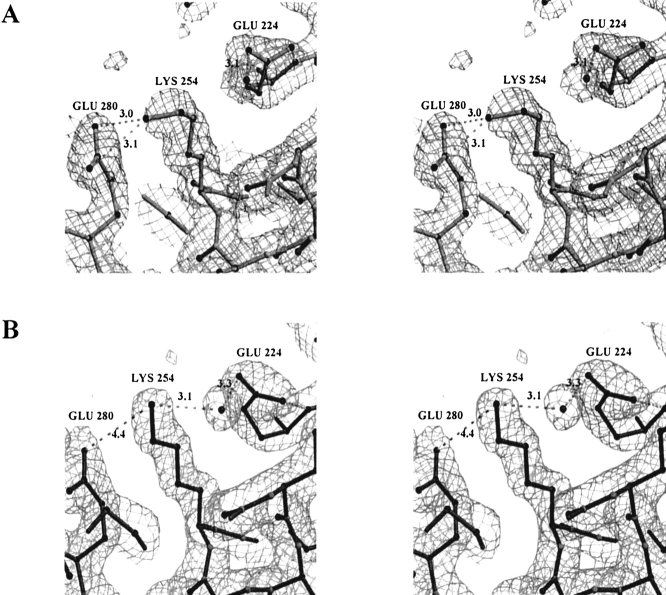
Fig. 5.
Two alternative conformations of Lys254 of the quadruple mutant of CbADH. (A) In subunit A, the ɛ-amino group of Lys254 points towards Glu280 to form a salt bridge; however neither hydrogen-bond connection nor electrostatic interaction between Lys254 and Glu224 is observed. (B) In subunit D, Lys254 is equally distanced from both Glu280 and Glu224; it interacts electrostatically with both of them and forms an additional hydrogen bond via a water molecule with Glu224. ′2Fo-Fc′ electron density map is contoured at 1.2σ.