Fig. 1.
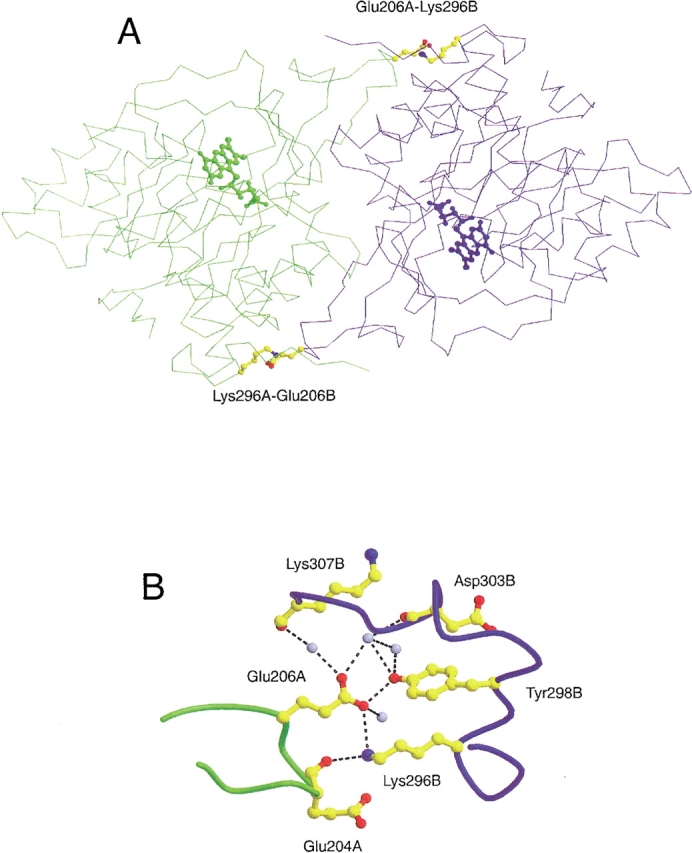
Position and environment of the E206-K296 salt bridges in the subunit interface of DHODA. (A) The two subunits are presented in different colors ("A", green, and "B", blue). The residues involved in formation of the intersubunit salt bridges, Glu206 and Lys296, are shown as balls and sticks in CPK colors, and the position of the active sites are indicated by their FMN cofactors. (B) The amino acid residues are represented as balls and sticks in CPK colors, and the colors of the peptide backbones have the same meaning as above. The light blue spheres represent water molecules, and the broken lines show hydrogen bonds with distances shorter than 3.4 Å. "A" and "B" specify the particular subunits as above. The distances from the carboxylate oxygen atom of Glu206 to the Nɛ of Lys296 and to the OH oxygen of Tyr298 are 3.1 Å and 2.6 Å, respectively. The programs Molscript (Kraulis 1991) and Raster 3D (Merritt and Bacon 1997) were used to prepare the figure, which is based on the coordinates from a recent structure determination of DHODA to 1.7 Å resolution (S. Nørager, S. Jensen, O. Björnberg, M. Oddosen, L. Leggio, K. Jensen, S. Larsen, in prep. pdb code number 1JUE.).
