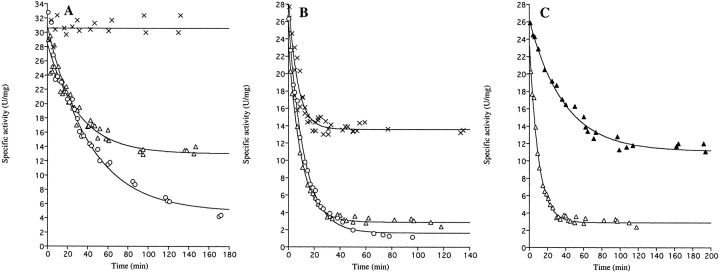
Fig. 2.
Time course of the loss of enzyme activity upon dilution of wild-type and E206K-K296E double mutant enzymes. At time t = 0, the enzymes were diluted in 0.1 M Tris-HCl, pH 8.0 (25°C). At the indicated times following dilution, samples were withdrawn for determination of enzymatic activity. (A) Wild-type DHODA diluted to 9.8 μM (×, Ao = 31 ± 1 U/mg, but calculations of the inactivation rate according to eq. 1 could not be made due to the stability of the enzyme at this high protein concentration); 0.098 μM (▵, Ao = 28.5 ± 0.5 U/mg, Ar = 13.0 ± 0.5 U/mg, kobs = 0.033 ± 0.003 min−1), or 0.029 μM (○, Ao = 31.0 ± 0.7 U/mg, Ar = 4.9 ± 0.7 U/mg, kobs = 0.024 ± 0.002 min−1). (B) The E206K-K296E double mutant enzyme diluted to 9.8 μM (×, Ao = 29.3 ± 0.9 U/mg, Ar = 13.6 ± 0.3 U/mg, kobs = 0.146 ± 0.015 min−1), 0.98 μM (▵, Ao = 24.1 ± 0.6 U/mg, Ar = 2.9 ± 0.1 U/mg, kobs = 0.103 ± 0.005 min−1), or 0.098 μM (○, Ao = 27.1 ± 0.4 U/mg, Ar = 1.6 ± 0.2 U/mg, kobs = 0.091 ± 0.003 min−1). (C) The E206K-K296E double mutant enzyme diluted to 0.98 μM in 0.1 M Tris-HCl pH 8.0 (▵, same curve as in (B) or in 0.1 M Tris-HCl pH 8.0 containing 0.15 M NaCl (▴, Ao = 26.3 ± 0.4 U/mg, Ar = 11.1 ± 0.3 U/mg, kobs = 0.026 ± 0.002 min−1). The curves are the best fits to the data points according to eq. 1. The kinetic parameters calculated according to eq. 1 are given in Table 2.