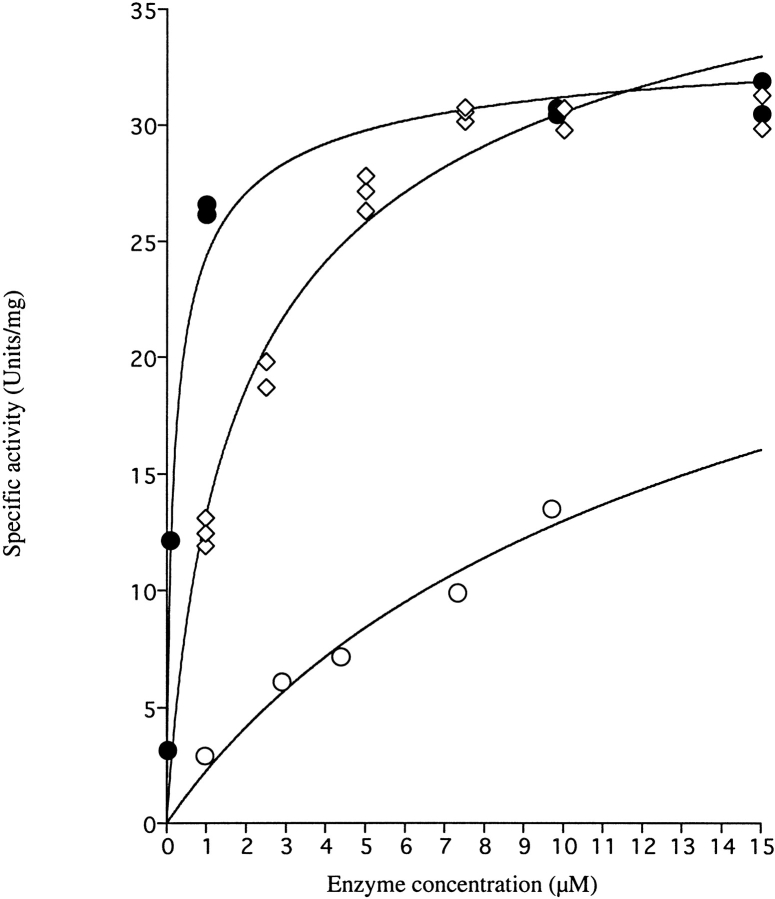
Fig. 3.
Remaining specific activity of DHODA as a function of enzyme concentration following dilution to different concentrations. •, wild-type DHODA diluted in 0.1 M Tris-HCl pH 8.0; ○, E206K-K296E double mutant diluted in 0.1 M Tris-HCl, pH 8.0; ⋄, E206K-K296E double mutant diluted in 0.1 M Tris-HCl, pH 8.0 containing 0.15 M NaCl. Following dilution at time t = 0, the samples were left to reach equilibrium for 200 min for the mutant DHODA and for 330 min for wild-type DHODA before assaying the remaining activity as described in Materials and Methods. The following parameters of the dissociation reaction were calculated according to eq. 3: Wild-type enzyme: KD = 0.26 ± 0.05 μM, SAMax = 35 ± 1 μmol min−1 mg−1; E206K-K296E double mutant: KD = 37 ± 22 μM, SAMax = 46 ± 17 μmol min−1 mg−1; E206K-K296E double mutant with 0.15M NaCl: KD = 3.5 ± 0.6 μM, SAMax = 46 ± 2 μmol min−1 mg−1.