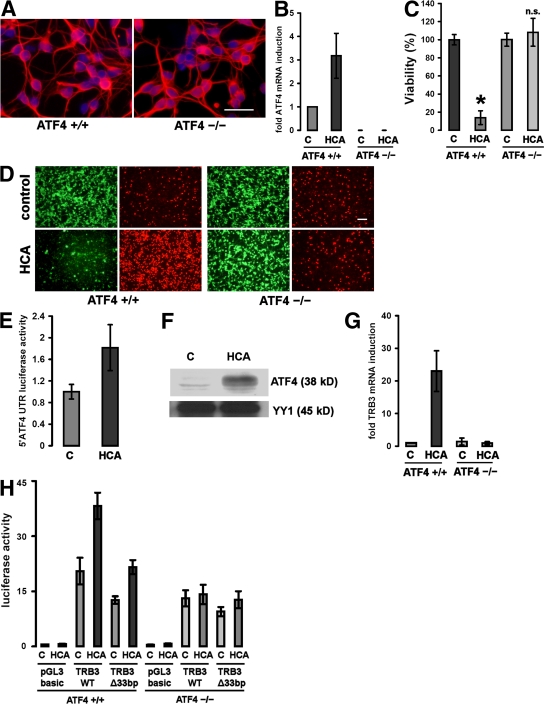
Figure 2.
Cortical neurons from ATF4−/− brains are resistant to oxidative stress–induced cell death. (A) Immunocytochemistry of cultured ATF4+/+ and ATF4−/− cortical neurons. Cells were stained with an antibody against MAP2 (which stains neural dendrites; red) and counterstained with Hoechst (blue). Bar, 50 μm. (B) Real-time PCR of ATF4 mRNA expression in ATF4+/+ and ATF4−/− neurons in response to treatment with 10 mM HCA. The value obtained from the ATF4+/+ control was arbitrarily defined as 1. Mean ± SD was calculated from three separate experiments. Each data point was performed in duplicate. (C) Cortical neuronal cultures (1 d in vitro) prepared from brains from ATF4+/+ and ATF4−/− embryos were treated with a vehicle control (shown as C) or 10 mM HCA. 24 h later, cell viability was determined using the MTT assay. The graph depicts mean (compared with control) ± SD calculated from data from five separate experiments (n = 27 ATF4+/+ and 73 ATF4−/−). *, P < 0.05 from ATF4+/+ untreated cultures by the Kruskal-Wallis test followed by Dunn's multiple comparisons test. The difference between treated and untreated ATF4−/− neurons was not significant (n.s.). (D) Representative live/dead assay displaying untreated and HCA-treated ATF4+/+ and ATF4−/− neurons. Bar, 50 μm. (E) ATF4+/+ neurons were transfected with a reporter plasmid (pGL3 backbone) containing the mouse ATF4 5′UTR and AUG fused to luciferase. Cortical neurons were cotransfected with a plasmid expressing Renilla to allow normalization for transfection efficiency. 24 h after transfection, neurons were treated with vehicle control (shown as C) or 10 mM HCA. Cells were harvested in luciferase assay buffer 12 h after the onset of treatment. Values were calculated from three separate experiments and are given as the ratio of luciferase and Renilla activities (mean ± SD; n = 3). The value for treatment with vehicle control was arbitrarily defined as 1. (F) Oxidative stress results in nuclear accumulation of ATF4 in cultured cortical neurons. 60 μg of nuclear extracts from cortical neurons treated with 10 mM HCA or vehicle control (shown as C) were separated using gel electrophoresis and immunodetected using an antibody against ATF4. YY1 was monitored as a loading control. (G) Real-time PCR of TRB3 mRNA expression in ATF4+/+ and ATF4−/− neurons in response to treatment with 10 mM HCA. The value obtained from the ATF4+/+ control was arbitrarily defined as 1. Mean ± SD was calculated from three separate experiments. Each data point was performed in duplicate. (H) ATF4+/+ and ATF4−/− neurons were transfected with a luciferase reporter plasmid (pGL3 backbone) containing a 2-kb fragment of the mouse TRB3 promoter (TRB3WT), with a mutant version of this promoter lacking the 33-bp ATF4 binding site (TRB3Δ33bp) or with the empty vector (pGL3 basic). Cortical neurons were cotransfected with a plasmid expressing Renilla to allow normalization for transfection efficiency. 24 h after transfection, neurons were treated with vehicle control (shown as C) or 10 mM HCA. Cells were harvested in luciferase assay buffer 12 h after the onset of treatment. Values are given as the ratio of luciferase and Renilla activities (mean ± SD) and were calculated from three separate experiments. Each data point was performed in duplicate. Values are given as the ratio of luciferase and Renilla activities (mean ± SD; n = 3). The value for empty pGL3 was arbitrarily defined as 1.