Figure 4. Ligands docked in the active site of Ado kinase.
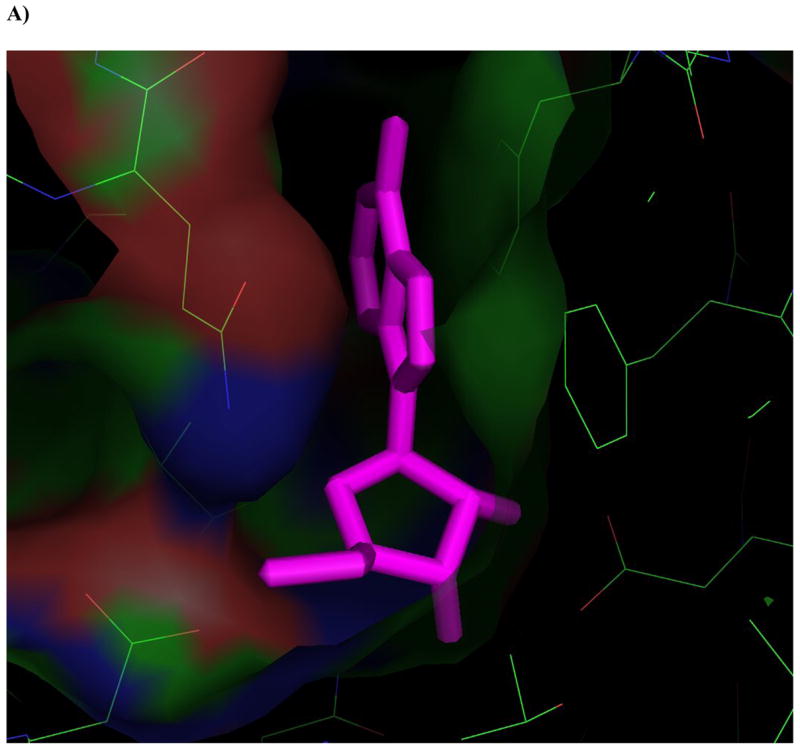
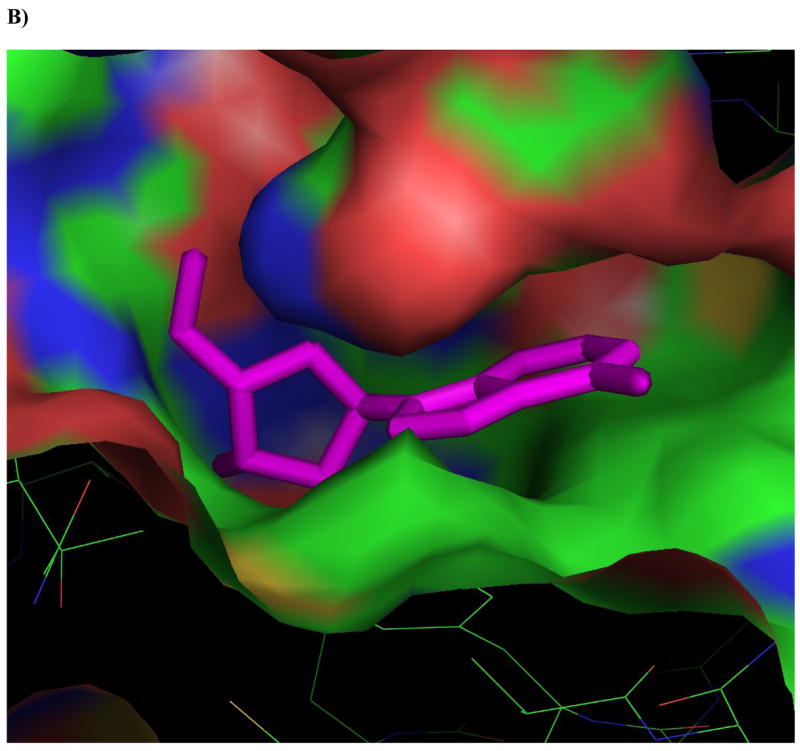
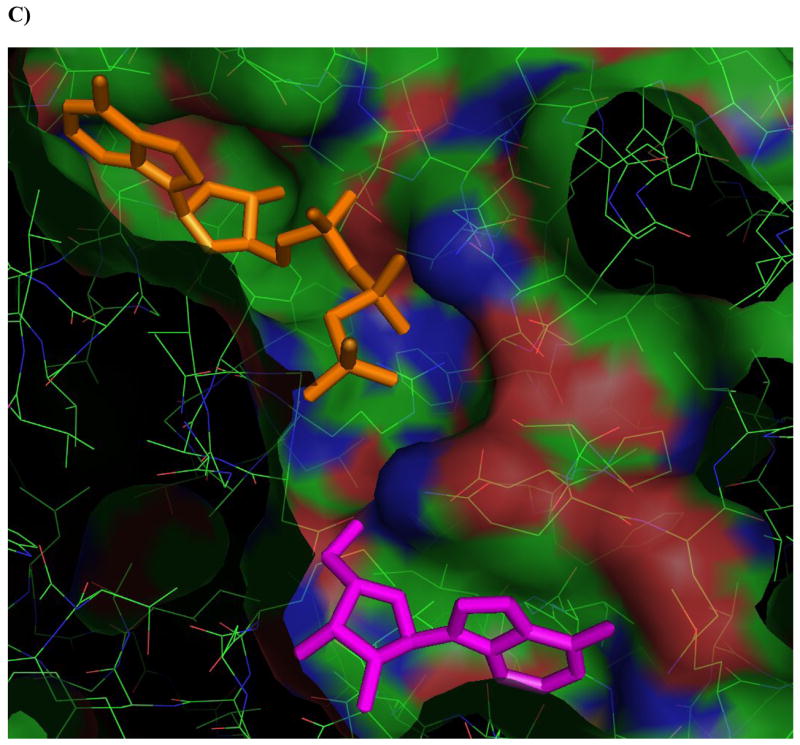
A) Adenosine (magenta) modeled into the active site from the perspective of the ribofuranosyl moiety. Phe 102 (right of center) forms the upper surface of the pocket where the ribofuranosyl moiety nests, while Asp 12 forms hydrogen bonds with the 2′ and 3′ hydroxyl groups (lower right). B) One of the better substrates, 9-[α-L-lyxofuranosyl]-adenine (34, Fig 2d) from a top-down perspective of looking downward from C1 and N7 of the adenine base. The area above this region is open and available for modifications. The yellow lining the pocket near the 2-position of the Ade base is Met 121, this is the pocket in which a 2-methyl or 2-fluoro group would rest. The 2′ and 3′-hydroxyl groups are clearly nested within the ribofuranosyl pocket, and there is plenty of room to rotate the 5′-hydroxyl so that it is trans to the adenine base, as in the case of 9-[α-L-lyxofuranosyl]-adenine (shown). C) This image depicts 9-[α-L-lyxofuranosyl]-adenine (magenta) modeled with AMP-PCP (orange), a non-hydrolysable form of ATP, in the active site of Ado kinase, as they would appear when Ado kinase is in a closed conformation. The γ-phosphate of AMP-PCP is nestled within the anion hole in the active site.
