Fig. 6.
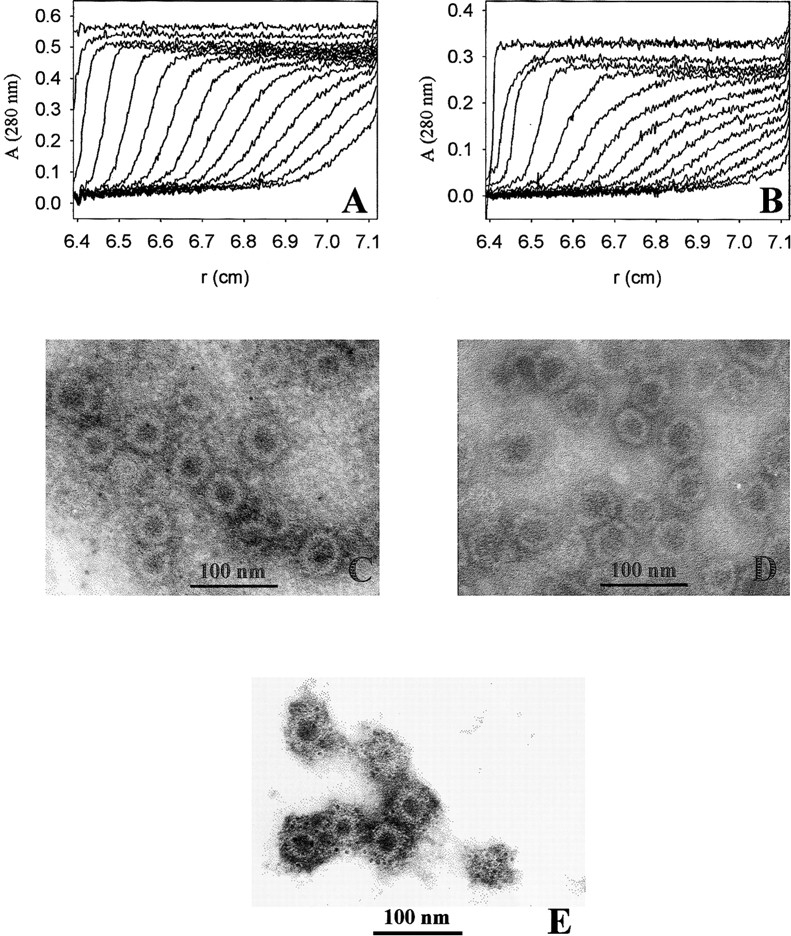
Formation of VLPs of VP1-Z. VP1-Z and wtVP1 at a protein concentration of ∼ 1 mg/mL was assembled to VLPs by dialysis against 20 mM Tris at pH 7.4, 200 mM NaCl, 5% glycerol, 0.5 M ammonium sulfate. The VLPs were analyzed by analytical ultracentrifugation. For electron microscopy the ammonium sulfate, which was present in the assembly buffer, was removed by dialysis. (A) Sedimentation velocity analysis of VP1-Z VLPs. An apparent S-value of 64 S was calculated. (B) Sedimentation velocity analysis of VLPs of wtVP1. The majority of the VLPs sedimented with sapp = 63 S. A small fraction assembled to larger particles (sapp = 96 S). VLPs of VP1-Z (C) and wtVP1 (D) were adsorbed to formvar-coated copper grids. The negative staining was performed using uranyl acetate. The electron micrograph was taken at a nominal magnification of 85,000×. (E) VLPs of VP1-Z adsorbed on Ni grids were coupled with gold-labeled antibody TU2. Afterward the complexes were stained with uranyl acetate. The electron micrograph was taken at a nominal magnification of 85,000×.
