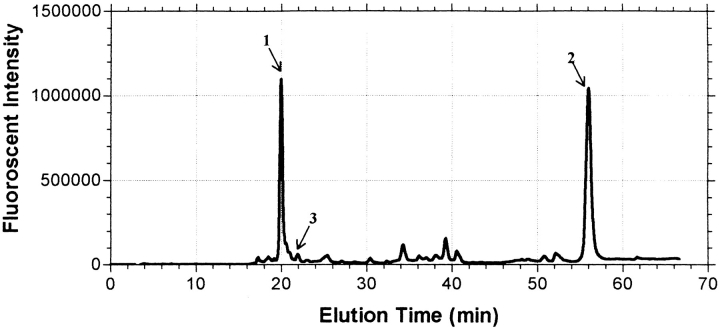
Fig. 11.
Relative amounts of fluorescent peptides observed following alkylation with IAEDANS. Incorporation of IAEDANS into hCG and its α- and β-subunits was monitored during subunit combination in the presence and absence of β-mercaptoethanol. Only trace amounts of IAEDANS were incorporated into these proteins in the absence of β-mercaptoethanol (not shown). In the presence of 1 and 3 mM β-mercaptoethanol, nearly all the incorporated AEDANS fluorophore was found equally in two peptides, namely β–Gly 75–Val-Asn-Pro-Val-Val-Ser-Tyr-Ala-Val-Ala-Leu-Ser-Cys-Gln-Cys-Ala-Leu-Cys–Arg 94 (peak 1) and β–Ser 96–Thr-Thr-Asp-Cys-Gly-Gly-Pro–Lys 104 (peak 2). The data shown here were obtained in the presence of 3 mM β-mercaptoethanol. MALDI-TOF analyses showed that only one molecule of AEDANS had been incorporated into each peptide. This is consistent with the idea that mild reduction disrupted the disulfide between the small seatbelt loop stabilized by β-subunit cysteines Cys 93 and Cys 100, making each available for alkylation by IAEDANS. Only trace amounts of AEDANS were incorporated into Cys 110 as seen by the relative absence of fluorescence in the peptide that contained this cysteine (Asp 105–His-Pro-Leu-Thr-Cys-Asp-Asp-Pro–Arg 114; peak 3). These results indicated that the acceleration of subunit combination at low concentrations of reducing agents was caused by the opening of the small seatbelt loop, a phenomenon that would increase the size of the hole in the β-subunit.