Fig. 4.
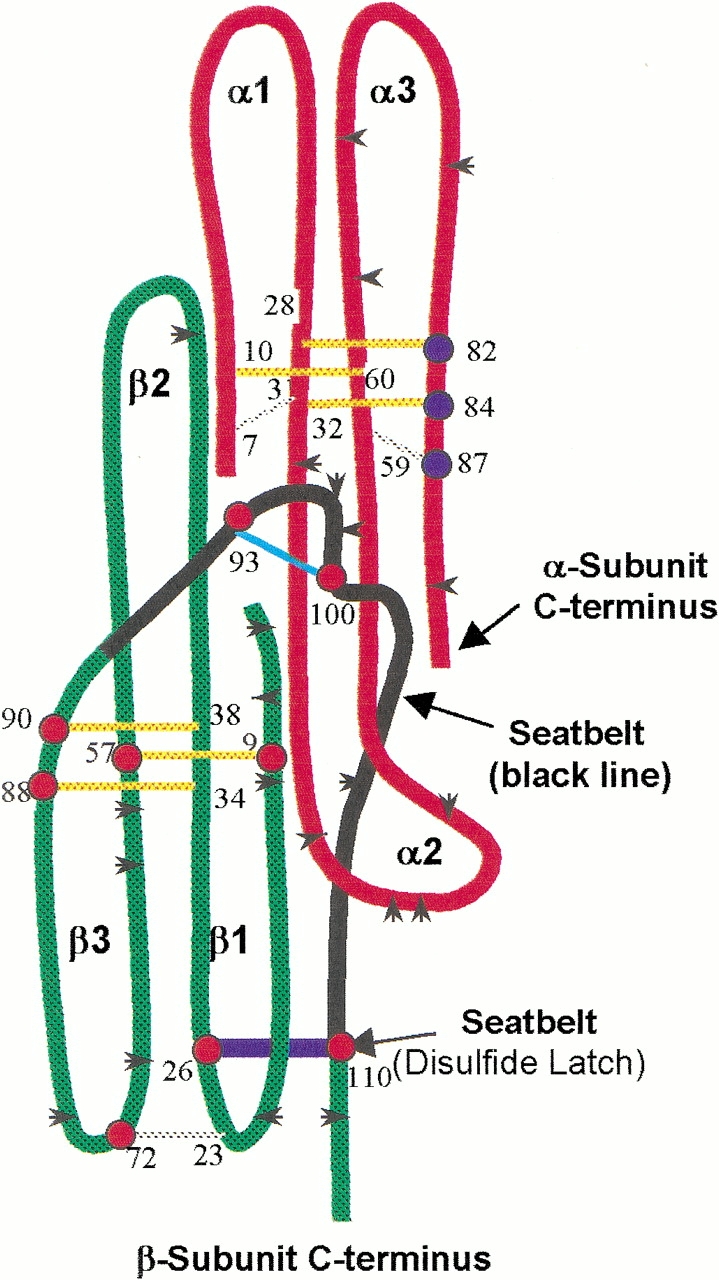
Cysteine residues studied by mass spectrometry. At least one cysteine member of all disulfides in both subunits except α7–α31 and α10–α60 were identified in tryptic peptides by mass spectrometry (α-subunit cysteines, blue dots; β-subunit cysteines, red dots). As summarized from five separate experiments, these cysteines were alkylated only after reduction, not during subunit combination. Thus, disruption of these disulfides was not required for subunit combination. Color scheme: black arrowheads, trypsin cleavage sites; red, α-subunit core; green, β-subunit core; black, β-subunit seatbelt; yellow, cysteine knot disulfides; cyan, seatbelt loop disulfide (Cys 93–Cys 100); blue, seatbelt latch disulfide (Cys 26–Cys 110); gray, remaining disulfides.
