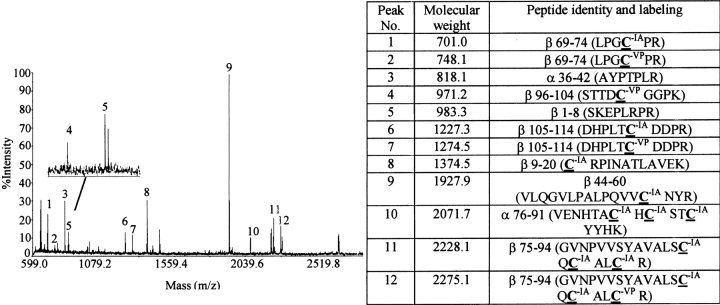
Fig. 9.
Identification of cysteines alkylated by VP during subunit combination in the presence of β-mercaptoethanol. hCG subunits were combined in the presence of 1 mM BME. VP (10 mM) was added within 5–10 sec after combination reaction had been initiated. Four hours later, when subunit combination was assumed to be complete, excess VP was removed by electrophoresis on a 15% SDS-polyacrylamide gel and proteins were electroeluted from a strip of gel containing the heterodimer and free subunits. Following denaturation and reduction of these proteins, cysteines that had not been alkylated by VP during subunit combination were alkylated by IA. Peptides produced by N-glycanase and trypsin digestion were subjected to MALDI-TOF to distinguish those that contained cysteines labeled by VP and IA. Shown here is a typical mass spectrum with the inset illustrating an enlarged region near peak 5. Highlighted amino acids in the table were labeled by VP or IA as noted. Note that cysteines from disulfides βC23–C72, βC93–C100 and βC26–C100 were labeled by both VP and IA, indicating that a portion of these disulfides had been reduced during subunit combination in the presence of 1 mM β-mercaptoethanol. The sequences of all peptides listed in the table were confirmed by LC/MS/MS.