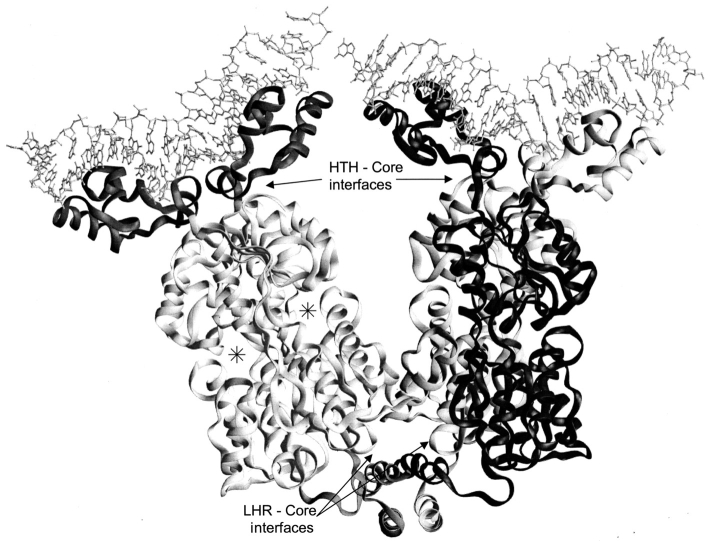
Fig. 1.
Structure of LacI. The LacI homotetramer is best described as a dimer of dimers (Friedman et al. 1995; Lewis et al. 1996). Monomers of the right dimer are black and gray to show how the N-terminal DNA-binding domains "cross over" the core domains of their partners. DNA is indicated at the top of the figure with balls and sticks. Each monomer comprises 360 amino acids. The first 60 amino acids are involved in DNA binding (Geisler and Weber 1977; Jovin et al. 1977; Ogata and Gilbert 1978; Khoury et al. 1991); a complete DNA binding site is composed of the two DNA-binding domains of a dimer (dark gray on left dimer). Residues 61–340 make up the core domain (light gray on left dimer; Platt et al. 1973; Files and Weber 1976), which contains the inducer binding site (one per monomer) and the monomer–monomer interface. The two inducer binding sites of the left dimer are indicated (*). The remaining residues (341–360) encompass an LHR sequence that forms the tetramerization domain (bottom of figure; Alberti et al. 1991, 1993; Chakerian et al. 1991; Chen et al. 1992a). This structure was generated from the pdb file 1lbg (Lewis et al. 1996).