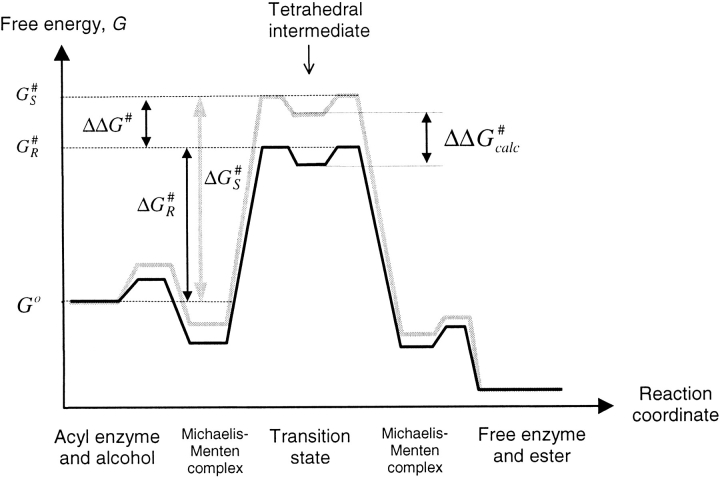
Fig. 2.
Free energy profile for the conversion of the serine hydrolase acyl enzyme and alcohol to the free enzyme and ester. The figure shows the energy profiles for the fast R enantiomer (dark) and the slow S enantiomer (light) of the substrate. The ground-state energy of the reactants, Go, is the same for both enantiomers. The activation free energies for R and S enantiomers are given by ΔG#R and ΔG#S, respectively, and the absolute free energies for the corresponding transition states are given by G#S and G#R. The difference in free energy between the transition state and its corresponding tetrahedral intermediate is negligible (Hu et al. 1998). Therefore, ΔΔG# can be approximated to the difference in absolute free energies between the tetrahedral intermediates of the R and S enantiomers, ΔΔG#calc.