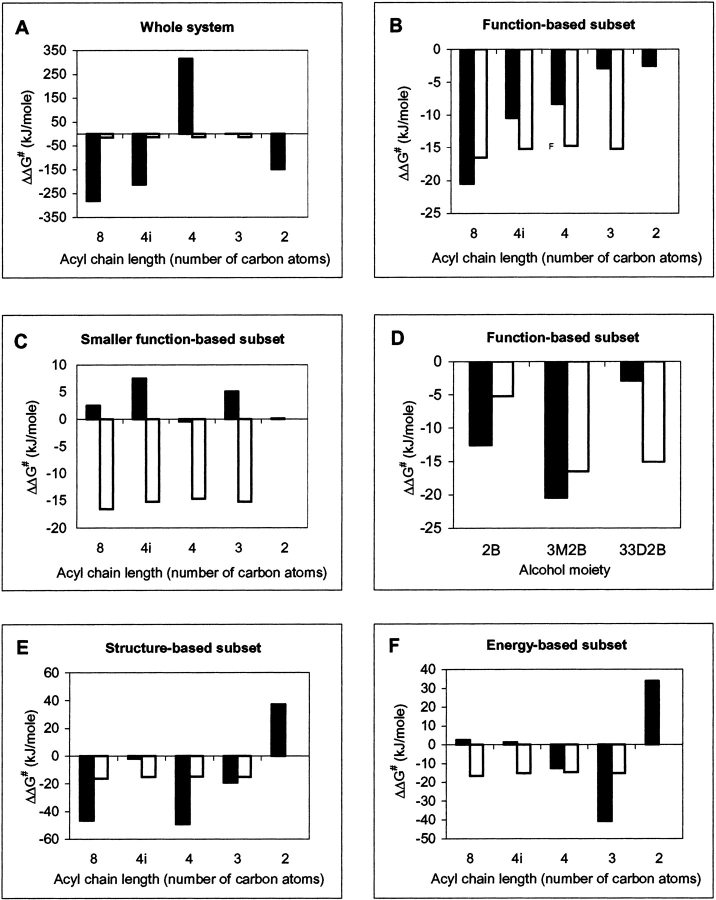
Fig. 4.
Comparison of calculated ΔΔG# values for various subsets (▪, black bars) with experimental values from Candida antarctica lipase B-catalyzed esterification in hexane (□, white bars). Parts A, B, C, E, and F refer to 3-methyl-2-butanol with variation in the acyl chain moiety. '4i' refers to iso-butanoyl; all other acyl moieties are straight chained. Part D refers to variation in the alcohol moiety (2B, 2-butanol; 3M2B, 3-methyl-2-butanol; 33D2B, 3,3-dimethyl-2-butanol); in each case octanoyl is used as the acyl moiety. The graphs denote potential energies for the following cases. (A) Whole of modeled system including waters. (B) and (D) Function-based subset describing the core structural elements of the transition state, as illustrated in Figure 5 ▶, for variation in acyl and alcohol moieties of the substrate, respectively. (C) Smaller function-based subset describing the substrate alcohol oxygen to His 224 hydrogen bond only, and consisting of the alcohol oxygen of the substrate, His 224:Nε and the corresponding hydrogen atom. (E) Structure-based subset consisting of full substrate and residues lining the active-site cavity, as given in Figure 6 ▶. (F) Energy-based subset consisting of the full substrate and those residues possessing a difference in interaction energy of ≥0.42 kJ mole−1 (0.1 kcal mole−1) between R and S enantiomers of the substrate.