Fig. 1.
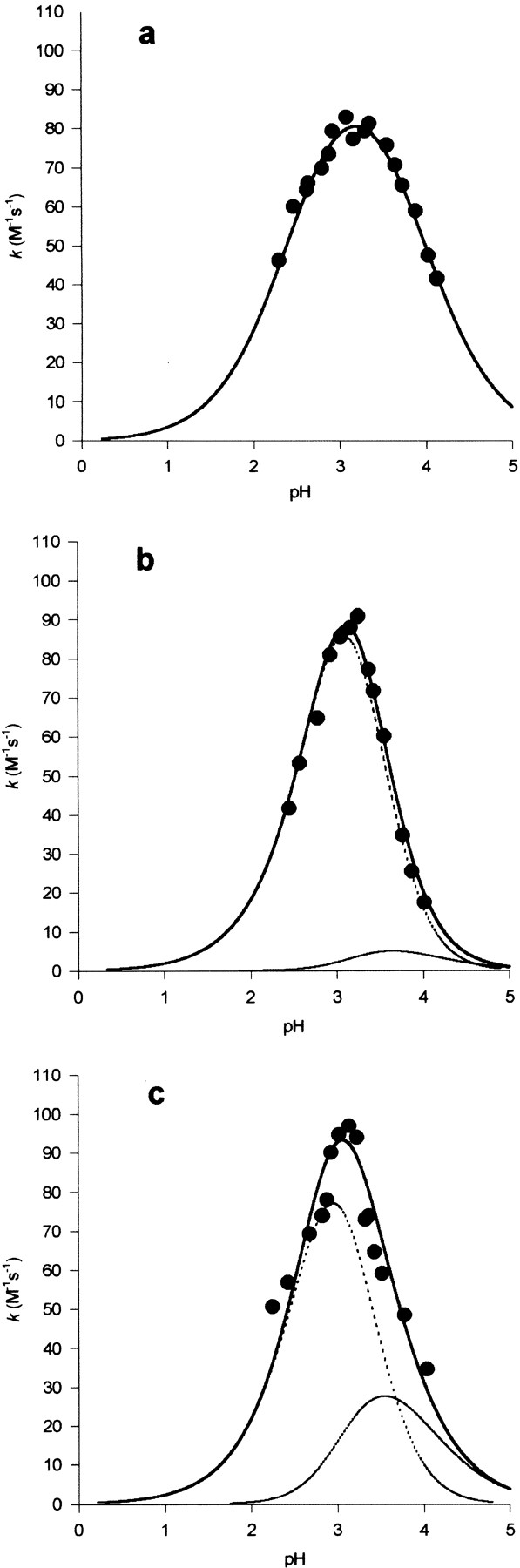
pH-dependence of the second-order rate constant (k) for the reactions at 25°C and I 0.1 M in aqueous buffers containing 1 mM EDTA and 2% (v/v) glycerol of 2PDS with (a) wild-type SK, (b) the C162S mutant, and (c) the K15M mutant. The points are each the mean of 3 determinations (SD ± ≤10% the mean). The continuous lines are theoretical for pH-dependent rate equations in a for 1 reactive protonic state and in b and c for 2 reactive protonic states and the following values of the characterizing parameters (pKa values and pH-independent rate constants, k̃, with values of the latter in M−1 sec−1): (a) pKI = 2.45, pKII = 3.9, k̃= 110; (b) pKI = 3.1, pKII = 3.2, pKIII = 3.9, k̃1 = 250, k̃2 = 10; (c) pKI = 3.0, pKII = 3.0, pKIII = 3.9, k̃1 = 240, k̃2 = 50. In b and c the broken lines are theoretical for the components of the pH-dependent rate equation corresponding to terms in k̃1 and k̃2. In a the broken line is coincident with the continuous line.
