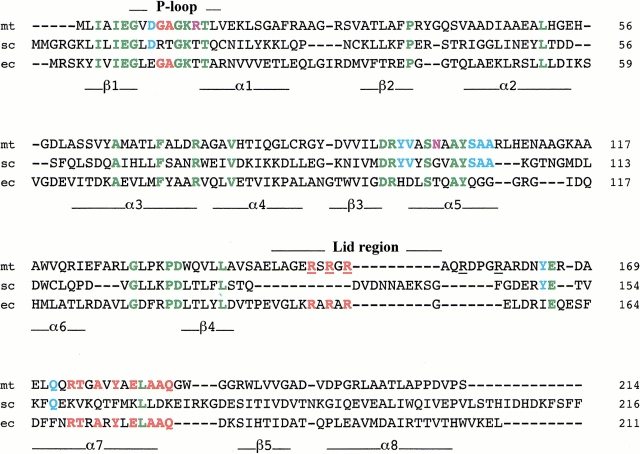
Fig. 1.
Alignment of the amino-acid sequences of TMPKmt (mt, this work) with TMPKy (sc) and TMPKec (ec). In green are identical residues in all three sequences. In light blue are amino acids common to TMPKy and TMPKmt and in red those that are common to TMPKec and TMPKmt to emphasise that TMPKmt might be considered as a chimeric sequence of TMPKy and TMPKec. Two positions occupied in TMPKmt by specific amino acids (Arg14 and Asn100) are shown in violet. Underlined arginine residues of TMPKmt are mentioned in the text. The secondary structure elements of TMPKy as well as the P-loop (involved in nucleoside di- or triphosphate binding) and the LID domain are shown under and over the alignment, respectively. The LID domains of TMPKy and TMPKec were not aligned as their backbone conformations differ greatly and do not superimpose.