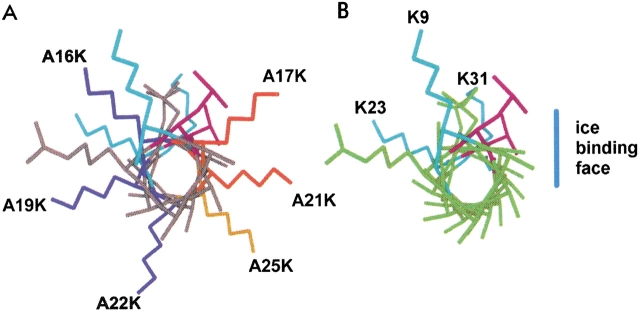
Fig. 3.
N-terminal view of SS-8 antifreeze protein from residues 9 to 31. Natural SS-8 Lys residues, K9, K23, and K31 are shown in light blue. (A) The position of A to K substitutions around SS-8. Dark blue Lys side chains (A16K, A19K, and A22K) indicate substitutions that have no effect on antifreeze activity. Red and orange side chains (A17K, A21K, and A25K) indicate severe and moderate effects to antifreeze activity, respectively. A25K (orange) interacts with ice crystals as seen by the bipyramidal morphology but fails to prevent ice crystal growth. (B) Wild-type SS-8 with natural Lys side chains indicated at positions 9, 23, and 31, and the deduced ice-binding plane indicated by the vertical blue line.