Fig. 1.
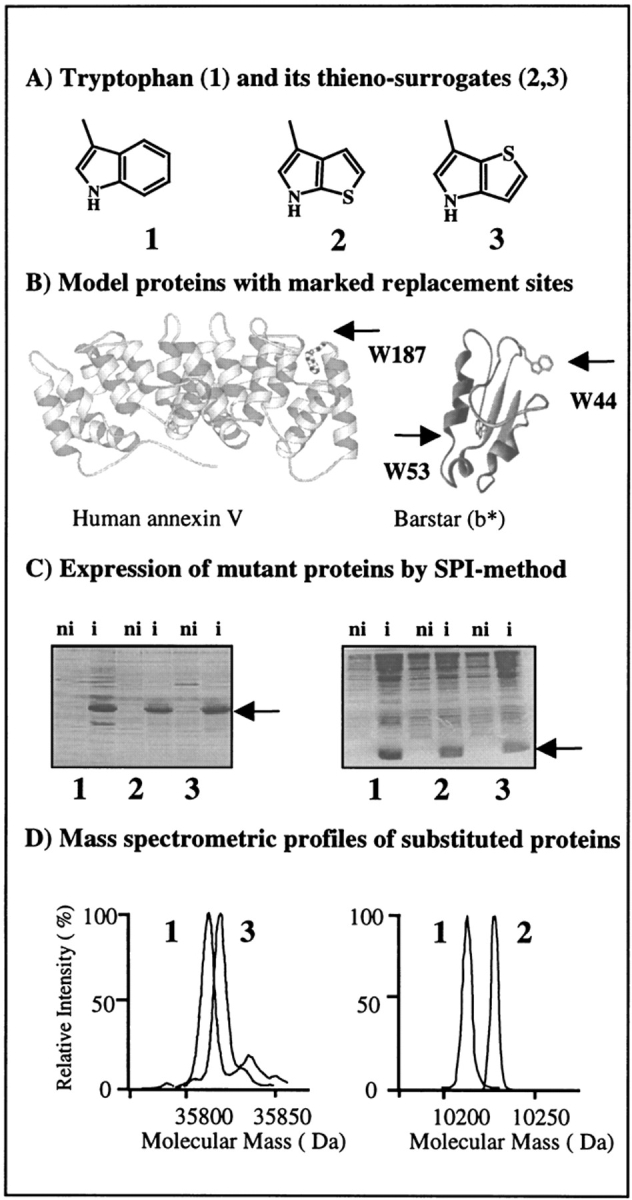
Flow chart for in vivo incorporation of tryptophan and β-(thienopyrrolyl)alanines by the SPI method into model proteins. (A) Structural representations of side chains of the canonical amino acid tryptophan (1) and its noncanonical thia-containing surrogates [2,3]Tpa (2) and [3,2]Tpa (3). (B) Ribbon plots of barstar (right) based on its PDB coordinates (Martin et al. 1999) with labeled locations of two tryptophan residues, W44 and W53, and side view of the AxV structure (left) with W187 buried in the hydrophobic pocket at the convex side of the molecule (Huber et al. 1990). Figures were prepared with MOLSCRIPT (Kraulis 1991). (C) Analysis of the expression profiles in cell lysates of E. coli ATCC49980 with overexpressed AxV (left) and b* (right) in the defined minimal medium with Trp (1), [2,3]Tpa (2), and [3,2]Tpa (3). (ni) Noninduced cells; (i) cells induced for protein expression. Arrows indicate position of overexpressed substituted proteins. (D) Analytical proof for substitutions by mass analyses of native and substituted proteins. Two different electrospray mass-spectrometric measurements were superimposed at the same mass-scale: (left) native and [3,2]Tpa-AxV; (right) wild-type and [2,3]Tpa-b*. Experimental conditions are described in Materials and Methods.
