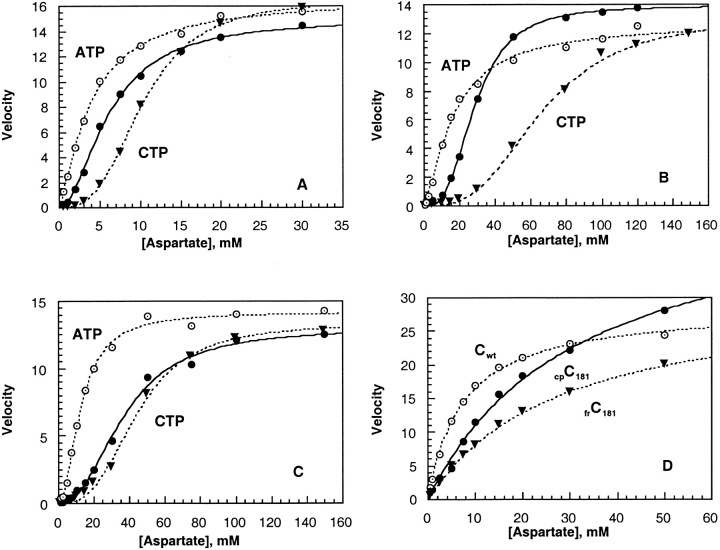
Fig. 4.
Enzyme activity of holoenzymes and C trimers containing fragmented and circularly permuted c chains. Assays were performed at 30°C with saturating [14C]carbamoyl phosphate (5 mM) in 50 mM MOPS buffer at pH 7.0, containing 0.2 mM EDTA and 2 mM β-mercaptoethanol. Results are given in the absence of effectors (black circle), in the presence of 2mM ATP (hollow circle), and in the presence of 0.5 mM CTP (black triangle). Enzyme activities are expressed as velocity in micromoles of carbamoyl-l-aspartate formed per hour per microgram of C trimer as a function of the concentration of aspartate. (A) H6ATCasewt; (B) frATCasec181; (C) cpATCasec181; (D) Trimers Cwt; frCc181; and cpCc181.