Fig. 2.

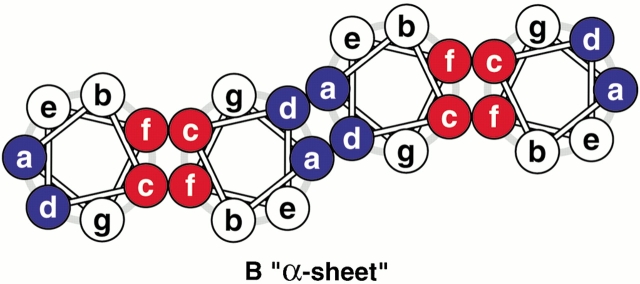
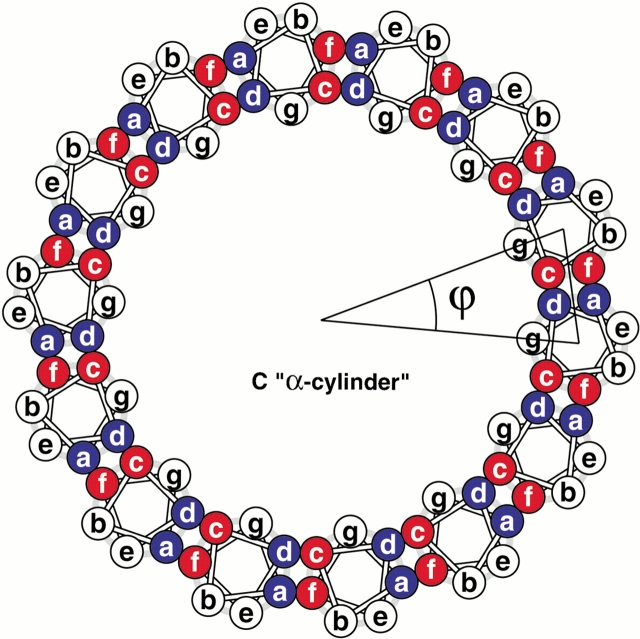
Construction of coiled-coil protein assemblies from bifaceted α-helices. (A) The lower part shows two heptad repeats aligned with a sequence offset of two residues. The two hydrophobic seams are highlighted and distinguished by the letters X and Y. The upper panel shows a 3.5-residue-per-turn helical wheel for the combined sequence. The resulting angular offset between the two hydrophobic seams, represented by the curved arrow and labelled θ, is ≈154°. (B) A syntypic assembly in which the a/d and c/f seams self-associate to form α-sheets. (C) An antitypic assembly in which the a/d and c/f faces associate. Such structures may close to form α-cylinders. The arc swept out by each helix (ϕ) is related to θ (ϕ = 180 − θ) and the number of helices required to close the cylinder (n) is given by n = 360 ÷ (180 − θ).