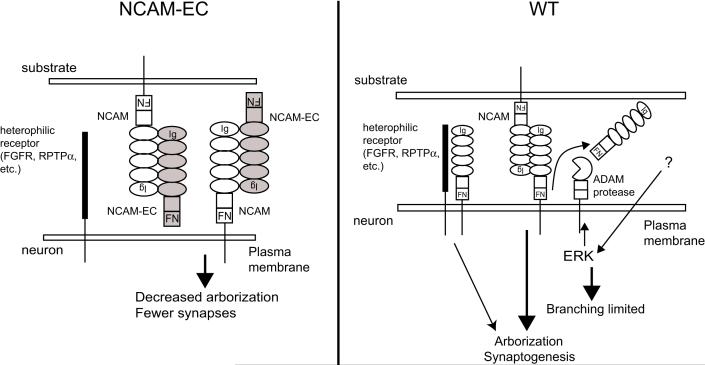
Figure 8. Model for the mechanism of action of the shed NCAM extracellular domain in interneuronal development.
Excess soluble NCAM-EC is depicted to interact with neuronal NCAM to inhibit NCAM-NCAM homophilic binding and heterophilic interactions with receptors such as the FGF receptor (FGFR) (Meiri 1998; Kiselyov 2003; Kiselyov 2005) or receptor protein tyrosine phosphatase alpha (RPTPα, (Bodrikov 2005)), thereby decreasing interneuron arborization to result in fewer perisomatic synapses. Alternatively, in other cell types where metalloprotease-induced soluble NCAM-EC promotes neurite growth (Meiri 1998; Hubschmann 2005; Kalus 2006), transgenic NCAM-EC might bind and inhibit this activity. In normal development, ERK MAP kinase, triggered by an external stimulus, activates an ADAM family protease to cleave NCAM and release NCAM-EC. This serves to limit interneuron branching promoted by NCAM homophilic or heterophilic binding.