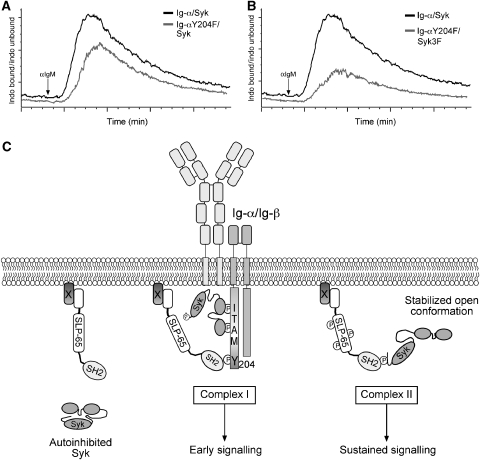
Figure 8.
Model for activation of BCR signalling. (A) Ig-α/Syk double-deficient cells were reconstituted to express Syk along with a BCR containing either Ig-αwt or Ig-αY204F, and Ca2+ flux was measured after crosslinking of the BCR with 10 μg/ml of anti-IgM. Baseline measurement was performed for 1 min before addition of anti-IgM and Ca2+ influx was measured for 5 min. (B) Ig-α/Syk double-deficient cells expressing a BCR containing Ig-αY204F along with either Sykwt, or Syk3F were compared for Ca2+ influx after BCR crosslinking as described above. (C) In resting cells, Syk exists in a closed autoinhibited conformation in a manner analogous to ZAP-70. Upon activation of the BCR, Syk binds to phosphorylated ITAMs and becomes activated. Activated Syk phosphorylates other neighbouring ITAM tyrosines and also the non-ITAM tyrosine of Ig-α. Phosphorylated Y204 serves as a docking site for the SH2 domain of SLP-65. Binding of SLP-65 to Ig-α brings it into close proximity of activated Syk (Complex I), which can then phosphorylate SLP-65 on multiple tyrosines leading to activation of many early signalling events. Activation of Syk leads to a conformation change in Syk, leading to exposure of its C terminus unmasking a phosphorylated tyrosine to which the SH2 domain of SLP-65 can bind. Binding of SLP-65 to Syk stabilizes Syk in an open active conformation (Complex II) and nucleates specific signalling complexes that activate many B-cell signalling pathways. Alternatively, the SLP-65/Syk complex could localize to specific locations in the cell and then mediate sustained signalling.