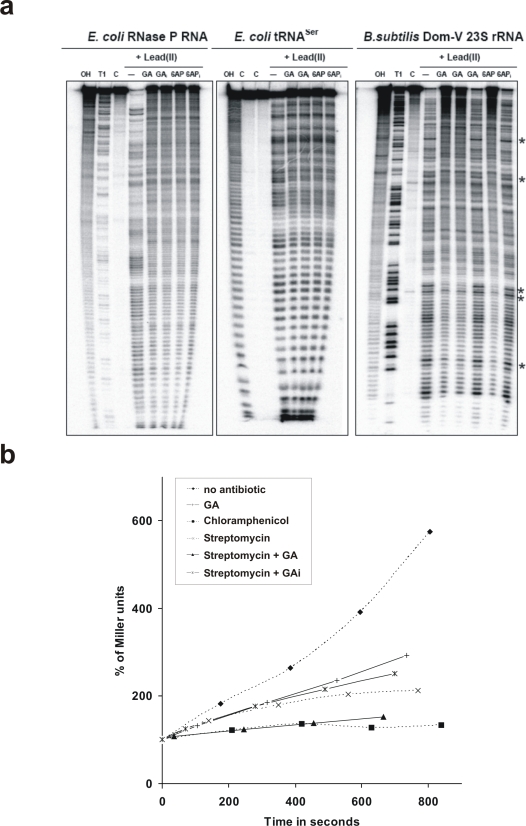
Figure 6. 6AP and GA specifically bind to some common positions on domain V of large rRNA and inhibit the in vivo protein folding activity of the ribosome in E. coli.
a. Chemical foot printing analysis of binding of 6AP and GA on various RNA. Asterisks indicate positions protected of degradation by Pb2+. Note that only 6AP and GA are able to protect some common positions specifically on Domain V of B. subtilis 23S rRNA (right gel) b. The increase in β-Gal activity was determined as a function of time in E. coli cells induced by IPTG and treated by the indicated compounds. Whereas both choramphenicol and streptomycin immediately and very efficiently inhibit translation, only chloramphenicol leads to an immediate inhibitory effect on increase in β-Gal activity, due to the fact that it also inhibits the protein folding activity of domain V of the large rRNA of the largest subunit of the ribosome. The increase observed in the presence of streptomycin corresponds to the folding by the ribosome of β-Gal translated just before translation inhibition by the antibiotic. This increase was inhibited by GA but not by GAi.