Figure 2.
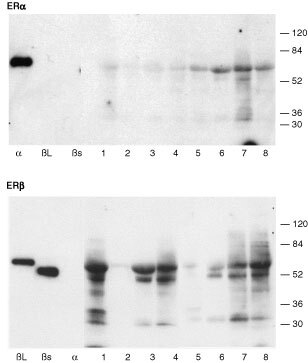
Western analysis of proteins extracted from breast cancer samples. Proteins were separated, blotted and incubated with antibodies directed against ERα (upper panel) or ERβ (lower panel). The anti-ERα antibody bound to recombinant hERα but not to recombinant hERβ (βs, βL). The anti-ERβ1 antibody bound to both long (βL) and short (βs) forms of recombinant hERβ but not to recombinant hERα (α). Proteins migrating with the same apparent molecular size as recombinant ERα (α, upper panel, arrowhead) were detected in all breast samples (lanes 1 to 8, note identical samples were used for both gels and are loaded in the same order). In sample numbers 6 and 7 additional lower molecular weight forms of ERα were present. Variable amounts of ERβ proteins were detected in the same samples. Proteins migrating with the same apparent molecular size as both long and short forms of ERβ proteins (arrowheads) were detected in breast sample numbers 1, 3, 4, 6, 7, 8; additional lower molecular weight variants were present in these same extracts but samples 2 and 5 lacked significant levels of ERβ.
