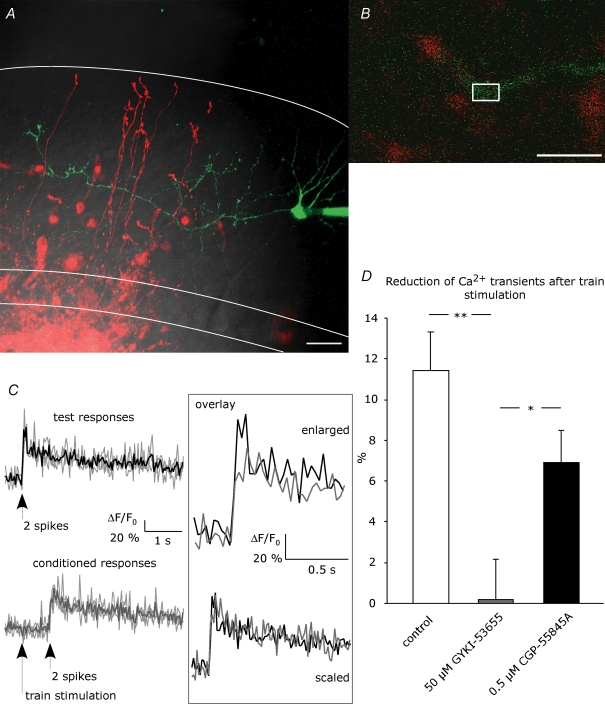
Figure 1. Ca2+ imaging in stellate cell axons.
A, a stellate cell was loaded with 100 μm Oregon Green 488 BAPTA-1 via the patch pipette. Granule cells were filled by electroporation achieved with repetitive stimulation in the granule cell layer with an extracellular pipette containing 500 μm Alexa Fluor 594. This picture is a z-projection, collecting the maximum intensity from 28 images taken in different focal planes with 1 μm intervals (scale bar = 20 μm). DIC, red and green channels are overlaid. White lines indicate Purkinje cell layer and rim of the molecular layer. Ca2+ signals were evoked by paired-pulse spiking of the stellate cells and were measured in ROIs set on the interneuron axon in proximity to granule cell axons, as depicted in an example in B (scale bar = 10 μm). C, example of test responses (black) and conditioned responses (grey) from a single hot spot. Fluorescence signals from 3 trials (depicted by faint lines) were analysed as ΔF/F0 and averaged. No Ca2+ signals were detected upon train stimulation. Scaling of the average traces revealed no change in the kinetics. D, reduction of peak amplitude of conditioned Ca2+ transients compared to test transients was examined in the absence of drug (control; number of ROIs, N = 53) and in the presence of AMPAR blocker (50 μm GYKI-53655, N = 42) or GABABR blocker (0.5 μm CGP-55845A, N = 41). Asterisks indicate statistical significance among series tested using ANOVA with Fisher's LSD post hoc test (*P < 0.05; **P < 0.01).