In a landmark article published 40 years ago Christoph Lüttgau and Hans Oetliker (Lüttgau & Oetliker, 1968) characterized quantitatively the action of caffeine on the activation of skeletal muscle. This paper, more than any others at that time, has created the impetus for the intense study of the (then) very poorly understood mechanism of excitation–contraction (EC) coupling in skeletal muscle. For example, in 1967 when the paper was written, there was no knowledge about the existence of the two major protagonists involved in signal transmission from the transverse (T-) tubular membrane to the intracellular Ca2+ stores, the dihydropyridine receptors (DHPRs)/voltage sensors in the membrane of the transverse tubular system to the ryanodine receptors (RyRs)/Ca2+ release channels in the sarcoplasmic reticulum (SR). The involvement of the DHPRs and RyRs in EC coupling and their close physical and functional relationships were only discovered in the late 1980s (for a review see Melzer et al. 1995). Moreover, it was also only in the late 1960s and early 1970s that the first reliable measurements of intracellular Ca2+ changes were reported in single muscle fibres following excitation using the Ca2+-sensitive photoprotein aequorin (Ridgway & Ashley, 1967; Ashley & Ridgway, 1970; Taylor et al. 1975).
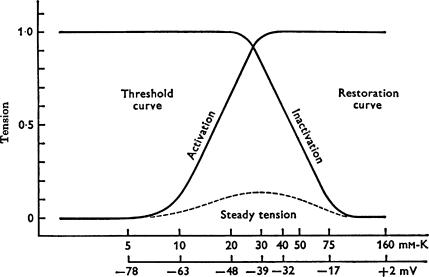
The detailed electrophysiological analysis by Lüttgau & Oetliker (1968) showed that extracellular application of caffeine induces different types of responses in single twitch skeletal muscle fibres of the frog, depending on its concentration. At low concentration (< 2 mm), caffeine does not cause depolarization of the surface membrane or spontaneous contracture, but rapidly shifts the voltage dependence of force tension activation towards more negative potentials and the steady-state voltage dependence of force tension inactivation towards more positive potentials. This creates a ‘window’ for a membrane potential between −50 and −20 mV where the fibre stays partially activated in the presence of 1.5 mm caffeine producing ‘steady’ force tension (Fig. 1). This is unlike the situation in the absence of caffeine when no ‘steady’ force tension was produced under the same conditions. Based on the results in Fig. 1, showing that the sensitivity of skeletal muscle fibres to low caffeine concentration is membrane potential dependent, Lüttgau and Oetliker concluded that the action of caffeine could be controlled by processes taking place ‘in the wall of the T-system’. This laid the foundation for the later introduction of the voltage-sensor concept by Schneider & Chandler (1973). At high concentrations (> 8 mm) caffeine induces a prolonged, maximal contracture in single frog fibres irrespective of the value of the membrane potential, which ultimately causes irreversible damage to the fibres (Lüttgau & Oetliker, 1968).
Figure 1. Threshold (‘activation’) and restoration (‘inactivation’) curves in 1.5 mM caffeine.
The X-axes refer to the extracellular [K+] and the corresponding calculated membrane potential. The dashed line represents the ‘steady tension’ in 1.5 mm caffeine measured at the corresponding membrane potential 20–40 s after the application of the drug (Fig. 14 from Lüttgau & Oetliker, 1968).
The observations made by Lüttgau & Oetliker (1968) with extracellular application of low caffeine concentrations led them to favour the T-tubular membrane as the site of caffeine action and this interpretation played a major role in shaping the direction of further research into the mechanism of EC coupling, particularly when studies on the SR showed that caffeine causes Ca2+ release from the SR (Weber & Herz, 1968; Endo et al. 1970). This apparent contradiction concerning the site of caffeine action could only be resolved after: (i) the development of the concept of ‘remote control’ of Ca2+ release from the SR by ‘voltage sensors’ in the tubular membranes (Schneider & Chandler, 1973); (ii) the SR Ca2+-release channels were found to be located in the junctional region between the SR cisternae and the tubular membrane (Block et al. 1988); and (iii) detailed studies of caffeine effects on RyR/Ca2+-release channel activity (Rousseau et al. 1988) and voltage sensor-dependent Ca2+ release from the SR (Klein et al. 1990; Shirokova & Ríos, 1996; Lamb et al. 2001).
With the knowledge that both activation of the voltage sensors in the T-system membrane and caffeine action on the RyR/Ca2+-release channels can open the same RyR/Ca2+-release channels and release Ca2+ from the SR, the main electrophysiological results obtained by Lüttgau & Oetliker (1968) with low caffeine concentration, summarized in Fig. 1, can be explained as follows. The presence of caffeine causes an increase in the open probability of the SR Ca2+-release channel (Rousseau et al. 1988) and renders it more sensitive to voltage sensor activation and physiological activators such as Ca2+ and ATP (Shirokova & Rios, 1996; Herrmann-Frank et al. 1999). This means that fewer voltage sensor activated Ca2+-release channels are needed to ensure a certain amount of Ca2+ released to reach the threshold for contractile apparatus activation. Consequently, the voltage-dependent Ca2+ release and force activation curves shift to more negative potentials while the force restoration (inactivation) curve moves to more positive potentials.
At higher concentrations, caffeine opens an increased number of SR Ca2+ release channels, raising the myoplasmic [Ca2+], which in turn, activates the contractile apparatus.
The ability of caffeine to increase the susceptibility of the SR Ca2+-release channels to activation by the voltage sensors and to reversibly open RyR/Ca2+-release channels and release Ca2+ from the SR makes it a most valuable tool to investigate specific aspects of the EC coupling mechanism under particular conditions (see review by Herrmann-Frank et al. 1999). Currently caffeine is routinely used in skeletal muscle research: (i) to distinguish between decrease in the amount of releasable pool of Ca2+ from the SR and some impaired coupling at a particular time during fatigue; (ii) to investigate specific aspects of the EC coupling at different levels of SR Ca2+-loading; (iii) to study the site of action of different drugs and modifiers of EC coupling using intact, ‘skinned’ or ‘cut fibre’ preparations; (iv) to investigate the properties of the SR Ca2+-release channels under various conditions using ‘skinned’ and ‘cut fibre’ preparations; (v) to examine the properties of the SR Ca2+ pump using ‘skinned’ fibres; and (vi) as a clinical diagnostic tool for determining patient susceptibility to malignant hyperthermia.
In conclusion, the 1968 paper of Lüttgau and Oetliker has been greatly stimulating for a generation of muscle physiologists interested in EC coupling and transformed a drug that is consumed daily by millions of people into a most valuable tool in muscle research.
References
- Ashley CC, Ridgway EB. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970;209:105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A, Lüttgau HC, Stephenson DG. Caffeine and excitation-contraction coupling in skeletal muscle: a stimulating story. J Muscle Res Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- Klein MG, Simon BJ, Schneider M. Effects of caffeine on calcium release from the sarcoplasmic reticulum in frog skeletal muscle fibers. J Gen Physiol. 1990;425:599–626. doi: 10.1113/jphysiol.1990.sp018120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA, Stephenson DG. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol. 2001;531:715–728. doi: 10.1111/j.1469-7793.2001.0715h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau HC, Oetliker H. The action of caffeine on the activation of the contractile mechanism in striated muscle fibres. J Physiol. 1968;194:51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Ridgway EB, Ashley CC. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967;29:229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Ladine J, Liu QY, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988;267:75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Shirokova N, Ríos E. Caffeine enhances intramembranous charge movement in frog skeletal muscle by increasing cytoplasmic Ca2+ concentration. J Physiol. 1996;493:341–356. doi: 10.1113/jphysiol.1996.sp021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Rüdel R, Blinks JR. Calcium transients in amphibian muscle. Fed Proc. 1975;34:1379–1381. [PubMed] [Google Scholar]
- Weber A, Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968;52:750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]